Chimeric Antigen Receptor-T Cells for Targeting Solid Tumors: Current Challenges and Existing Strategies
- PMID: 31363930
- PMCID: PMC6790340
- DOI: 10.1007/s40259-019-00368-z
Chimeric Antigen Receptor-T Cells for Targeting Solid Tumors: Current Challenges and Existing Strategies
Abstract
Chimeric antigen receptor-T cells (CAR-Ts) are an exciting new cancer treatment modality exemplified by the recent regulatory approval of two CD19-targeted CAR-T therapies for certain B cell malignancies. However, this success in the hematological setting has yet to translate to a significant level of objective clinical responses in the solid tumor setting. The reason for this lack of translation undoubtedly lies in the substantial challenges raised by solid tumors to all therapies, including CAR-T, that differ from B cell malignancies. For instance, intravenously infused CAR-Ts are likely to make rapid contact with cancerous B cells since both tend to reside in the same vascular compartments within the body. By contrast, solid cancers tend to form discrete tumor masses with an immune-suppressive tumor microenvironment composed of tumor cells and non-tumor stromal cells served by abnormal vasculature that restricts lymphocyte infiltration and suppresses immune function, expansion, and persistence. Moreover, the paucity of uniquely and homogeneously expressed tumor antigens and inherent plasticity of cancer cells provide major challenges to the specificity, potency, and overall effectiveness of CAR-T therapies. This review focuses on the major preclinical and clinical strategies currently being pursued to tackle these challenges in order to drive the success of CAR-T therapy against solid tumors.
Conflict of interest statement
Lorraine Springuel, Caroline Lonez, Bertrand Alexandre, David E. Gilham, Anne Flament, and Frédéric F. Lehmann are employees of Celyad SA. Mateusz Opyrchal has consulting agreements with Novartis and AstraZeneca, and has received research funding from Pfizer and Bayer. Eric Van Cutsem reports participation in advisory boards for AstraZeneca, Bayer, Bristol-Myers Squibb, Celgene, Lilly, Merck Sharp & Dohme, Merck KGaA, Novartis, Roche, and Servier and research grants from Amgen, Bayer, Boehringer Ingelheim, Celgene, Ipsen, Lilly, Roche, Merck Sharp & Dohme, Merck KGaA, Novartis, Roche, and Servier paid to his institution (Cliniques Universitaires Saint-Luc) outside the submitted work. Jean-Pascal H. Machiels, Marc Van Den Eynde, Hans Prenen, Alain Hendlisz, Eric Van Cutsem, Leila Shaza, Javier Carrasco, Jean-Luc Canon, Mateusz Opyrchal, Kunle Odunsi, and Sylvie Rottey are investigators on Celyad’s sponsored trials.
Figures
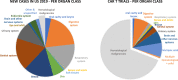
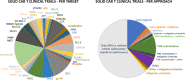
References
-
- Leick MB, Maus MV. CAR-T cells beyond CD19, UnCAR-Ted territory. Am J Hematol. 2019;94:S34–S41. - PubMed
-
- Havard R, Stephens DM. Anti-CD19 chimeric antigen receptor T cell therapies: harnessing the power of the immune system to fight diffuse large B cell lymphoma. Curr Hematol Malig Rep. 2018;13:534–542. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

