Sensitivity of 18F-fluorodihydrotestosterone PET-CT to count statistics and reconstruction protocol in metastatic castration-resistant prostate cancer
- PMID: 31363939
- PMCID: PMC6667590
- DOI: 10.1186/s13550-019-0531-8
Sensitivity of 18F-fluorodihydrotestosterone PET-CT to count statistics and reconstruction protocol in metastatic castration-resistant prostate cancer
Abstract
Objectives: Whole body [18F]-fluorodihydrotestosterone positron emission tomography ([18F]FDHT PET) imaging directly targets the androgen receptor and is a promising prognostic and predictive biomarker in metastatic castration-resistant cancer (mCRPC). To optimize [18F]FDHT PET-CT for diagnostic and response assessment purposes, we assessed how count statistics and reconstruction protocol affect its accuracy, repeatability, and lesion detectability.
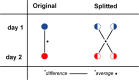
Methods: Whole body [18F]FDHT PET-CT scans were acquired on an analogue PET-CT on two consecutive days in 14 mCRPC patients harbouring a total of 336 FDHT-avid lesions. Images were acquired at 45 min post-injection of 200 MBq [18F]FDHT at 3 min per bed position. List-mode PET data were split on a count-wise basis, yielding two statistically independent scans with each 50% of counts. Images were reconstructed according to current EANM Research Ltd. (EARL1, 4 mm voxel) and novel EARL2 guidelines (4 mm voxel + PSF). Per lesion, we measured SUVpeak, SUVmax, SUVmean, and contrast-to-noise ratio (CNR). SUV was normalized to dose per bodyweight as well as to the parent plasma input curve integral. Variability was assessed with repeatability coefficients (RCs).
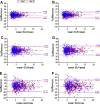
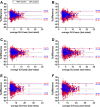
Results: Count reduction increased liver coefficient of variation from 9.0 to 12.5% and from 10.8 to 13.2% for EARL1 and EARL2, respectively. SUVs of EARL2 images were 12.0-21.7% higher than EARL1. SUVs of 100% and 50% count data were highly correlated (R2 > 0.98; slope = 0.97-1.01; ICC = 0.99-1.00). Intrascan variability was volume-dependent, and count reduction resulted in higher intrascan variability for EARL2 than EARL1 images. Intrascan RCs were lowest for SUVmean (8.5-10.6%), intermediate for SUVpeak (12.0-16.0%), and highest for SUVmax (17.8-22.2%). Count reduction increased test-retest variance non-significantly (p > 0.05) for all SUV types and normalizations. For SUVpeak at 50% of counts, RCs remained < 30% when small lesions were excluded. Splitting data reduced CNR by median 4.6% (interquartile range 1.2-8.7%) and 4.6% (interquartile range 1.2-8.7%) for EARL1 and EARL2 images, respectively.
Conclusions: Reducing [18F]FDHT PET acquisition time from 3 min to 1.5 per bed position resulted in a repeatability of SUVpeak (bodyweight) remaining ≤ 30%, which is generally acceptable for response monitoring purposes. However, EARL2 reconstruction was more affected, especially for SUVmax whose repeatability tended to exceed 30%. Lesion detectability was only slightly impaired by reducing acquisition time, which might not be clinically relevant in mCRPC.
Keywords: Count statistics; PET-CT; Reconstruction; [18F]-fluordihydrotestosterone; mCRPC.
Conflict of interest statement
DH is an employee of Philips Healthcare. The other authors declare that they have no competing interests.
Figures




Similar articles
-
[18F]-FDHT PET for the Imaging of Androgen Receptor in Prostate and Breast Cancer: A Systematic Review.Diagnostics (Basel). 2023 Aug 7;13(15):2613. doi: 10.3390/diagnostics13152613. Diagnostics (Basel). 2023. PMID: 37568977 Free PMC article. Review.
-
Reproducibility and Repeatability of Semiquantitative 18F-Fluorodihydrotestosterone Uptake Metrics in Castration-Resistant Prostate Cancer Metastases: A Prospective Multicenter Study.J Nucl Med. 2018 Oct;59(10):1516-1523. doi: 10.2967/jnumed.117.206490. Epub 2018 Apr 6. J Nucl Med. 2018. PMID: 29626121 Free PMC article. Clinical Trial.
-
Quantitative and clinical implications of the EARL2 versus EARL1 [18F]FDG PET-CT performance standards in head and neck squamous cell carcinoma.EJNMMI Res. 2023 Oct 25;13(1):91. doi: 10.1186/s13550-023-01042-w. EJNMMI Res. 2023. PMID: 37878160 Free PMC article.
-
Assessment of Simplified Methods for Quantification of 18F-FDHT Uptake in Patients with Metastatic Castration-Resistant Prostate Cancer.J Nucl Med. 2019 Sep;60(9):1221-1227. doi: 10.2967/jnumed.118.220111. Epub 2019 Mar 8. J Nucl Med. 2019. PMID: 30850488 Free PMC article.
-
18F-NaF PET/CT of Obese Patients on a Lutetium-Yttrium Oxyorthosilicate PET/CT System: Patient Dosimetry, Optimization of Injected Activity, and Acquisition Time.J Nucl Med Technol. 2021 Jun;49(2):150-155. doi: 10.2967/jnmt.120.258137. Epub 2020 Dec 30. J Nucl Med Technol. 2021. PMID: 33380519 Review.
Cited by
-
Repeatability of Quantitative 18F-DCFPyL PET/CT Measurements in Metastatic Prostate Cancer.J Nucl Med. 2020 Sep;61(9):1320-1325. doi: 10.2967/jnumed.119.236075. Epub 2020 Jan 10. J Nucl Med. 2020. PMID: 31924729 Free PMC article. Clinical Trial.
-
[18F]-FDHT PET for the Imaging of Androgen Receptor in Prostate and Breast Cancer: A Systematic Review.Diagnostics (Basel). 2023 Aug 7;13(15):2613. doi: 10.3390/diagnostics13152613. Diagnostics (Basel). 2023. PMID: 37568977 Free PMC article. Review.
-
PET imaging of brain aromatase in humans and rhesus monkeys by 11C-labeled cetrozole analogs.Sci Rep. 2021 Dec 8;11(1):23623. doi: 10.1038/s41598-021-03063-8. Sci Rep. 2021. PMID: 34880350 Free PMC article.
-
Ezrin expression in circulating tumor cells is a predictor of prostate cancer metastasis.Bioengineered. 2022 Feb;13(2):4076-4084. doi: 10.1080/21655979.2021.2014710. Bioengineered. 2022. PMID: 35156523 Free PMC article.
-
Potential Targets Other Than PSMA for Prostate Cancer Theranostics: A Systematic Review.J Clin Med. 2021 Oct 24;10(21):4909. doi: 10.3390/jcm10214909. J Clin Med. 2021. PMID: 34768432 Free PMC article. Review.
References
-
- Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152–160. doi: 10.1016/S1470-2045(14)71205-7. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

