Anti-endotoxin Properties of Polymyxin B-immobilized Fibers
- PMID: 31364085
- PMCID: PMC7123644
- DOI: 10.1007/978-3-030-16373-0_19
Anti-endotoxin Properties of Polymyxin B-immobilized Fibers
Abstract
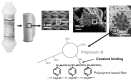
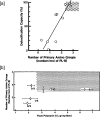
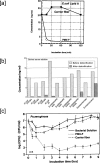
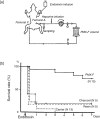
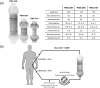
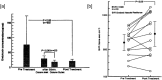
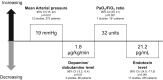
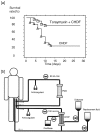
Polymyxin B is an antibiotic that shows strong bactericidal activity against Gram-negative bacteria, by binding to and inactivating endotoxin. Systemic administration of polymyxin B in humans is restricted because of its nephrotoxicity and neurotoxicity, and this compound was therefore considered a strong candidate ligand for the extracorporeal selective adsorption of circulating endotoxin in the blood. Toraymyxin® is a direct hemoperfusion column that uses polymyxin B attached to an insoluble carrier to bind endotoxin in the blood. In 1994, the Japanese National Health Insurance system approved the use of Toraymyxin for the treatment of endotoxemia and septic shock.In this chapter, we will review the development, clinical use, and efficacy of Toraymyxin, examine the structure of the Toraymyxin column, and comment on the current position of Toraymyxin in the treatment of severe sepsis and septic shock. We will also highlight some potential new applications of Toraymyxin for pulmonary diseases.
Keywords: Endotoxemia; Lipopolysaccharide; Polymyxin; Septic shock; Toraymyxin.
Figures





Similar articles
-
Endotoxin adsorption: Direct hemoperfusion with the polymyxin B-immobilized fiber column (PMX).Transfus Apher Sci. 2017 Oct;56(5):682-688. doi: 10.1016/j.transci.2017.08.015. Epub 2017 Aug 31. Transfus Apher Sci. 2017. PMID: 28923774 Review.
-
Extracorporeal endotoxin removal for the treatment of sepsis: endotoxin adsorption cartridge (Toraymyxin).Ther Apher Dial. 2003 Feb;7(1):108-14. doi: 10.1046/j.1526-0968.2003.00005.x. Ther Apher Dial. 2003. PMID: 12921125 Review.
-
History and current status of polymyxin B-immobilized fiber column for treatment of severe sepsis and septic shock.Ann Gastroenterol Surg. 2017 Jul 4;1(2):105-113. doi: 10.1002/ags3.12015. eCollection 2017 Jun. Ann Gastroenterol Surg. 2017. PMID: 29863114 Free PMC article. Review.
-
Clinical effects of direct hemoperfusion using a polymyxin-B immobilized column in solid organ transplanted patients with signs of severe sepsis and septic shock. A pilot study.Int J Artif Organs. 2007 Oct;30(10):915-22. doi: 10.1177/039139880703001009. Int J Artif Organs. 2007. PMID: 17992653 Clinical Trial.
-
Clinical effects of use polymyxin B fixed on fibers in liver transplant patients with severe sepsis or septic shock.Transplant Proc. 2007 Jul-Aug;39(6):1953-5. doi: 10.1016/j.transproceed.2007.05.064. Transplant Proc. 2007. PMID: 17692664
Cited by
-
Creating a Vaccine-like Supplement against Respiratory Infection Using Recombinant Bacillus subtilis Spores Expressing SARS-CoV-2 Spike Protein with Natural Products.Molecules. 2023 Jun 26;28(13):4996. doi: 10.3390/molecules28134996. Molecules. 2023. PMID: 37446658 Free PMC article.
-
Anti-Endotoxin 9-Meric Peptide with Therapeutic Potential for the Treatment of Endotoxemia.J Microbiol Biotechnol. 2021 Jan 28;31(1):25-32. doi: 10.4014/jmb.2011.11011. J Microbiol Biotechnol. 2021. PMID: 33263333 Free PMC article.
-
Application of Adsorptive Blood Purification Techniques during Cardiopulmonary Bypass in Cardiac Surgery.Oxid Med Cell Longev. 2022 May 25;2022:6584631. doi: 10.1155/2022/6584631. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35663201 Free PMC article. Review.
-
Ultra-High Packing Density Next Generation Microtube Array Membrane for Absorption Based Applications.Membranes (Basel). 2021 Apr 8;11(4):273. doi: 10.3390/membranes11040273. Membranes (Basel). 2021. PMID: 33917933 Free PMC article.
-
Bacterial lipopolysaccharide-induced endothelial activation and dysfunction: a new predictive and therapeutic paradigm for sepsis.Eur J Med Res. 2023 Sep 12;28(1):339. doi: 10.1186/s40001-023-01301-5. Eur J Med Res. 2023. PMID: 37700349 Free PMC article. Review.
References
-
- Umeda M, Niwa M, Yamagami S, Kishimot T, Maekawa M, Sawada Y. Novel endotoxin adsorbing materials, polymyxin-sepharose and polyporous polyethylene membrane for removal of endotoxin from dialysis systems. Biomater Artif Cells Artif Organs. 1990;18(4):491–497. doi: 10.3109/10731199009119623. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

