Cellular cholesterol modifies flow-mediated gene expression
- PMID: 31364378
- PMCID: PMC6843042
- DOI: 10.1152/ajprenal.00196.2019
Cellular cholesterol modifies flow-mediated gene expression
Abstract
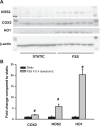
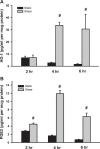
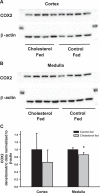
Downregulation of heme oxygenase-1 (HO-1), cyclooxygenase-2 (COX2), and nitric oxide synthase-2 (NOS2) in the kidneys of Dahl rodents causes salt sensitivity, while restoring their expression aids in Na+ excretion and blood pressure reduction. Loading cholesterol into collecting duct (CD) cells represses fluid shear stress (FSS)-mediated COX2 activity. Thus, we hypothesized that cholesterol represses flow-responsive genes necessary to effectuate Na+ excretion. To this end, CD cells were used to test whether FSS induces these genes and if cholesterol loading represses them. Mice fed either 0% or 1% cholesterol diet were injected with saline, urine volume and electrolytes were measured, and renal gene expression determined. FSS-exposed CD cells demonstrated increases in HO-1 mRNA by 350-fold, COX2 by 25-fold, and NOS2 by 8-fold in sheared cells compared with static cells (P < 0.01). Immunoblot analysis of sheared cells showed increases in HO-1, COX2, and NOS2 protein, whereas conditioned media contained more HO-1 and PGE2 than static cells. Cholesterol loading repressed the sheared mediated protein abundance of HO-1 and NOS2 as well as HO-1 and PGE2 concentrations in media. In cholesterol-fed mice, urine volume was less at 6 h after injection of isotonic saline (P < 0.05). Urinary Na+ concentration, urinary K+ concentration, and osmolality were greater, whereas Na+ excretion was less, at the 6-h urine collection time point in cholesterol-fed versus control mice (P < 0.05). Renal cortical and medullary HO-1 (P < 0.05) and NOS2 (P < 0.05) mRNA were repressed in cholesterol-fed compared with control mice. Cholesterol acts to repress flow induced natriuretic gene expression, and this effect, in vivo, may contribute to renal Na+ avidity.
Keywords: blood pressure; collecting duct; flow; shear.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures










Comment in
-
Cholesterol may not have a special place in kidneys.Am J Physiol Renal Physiol. 2019 Nov 1;317(5):F1169-F1170. doi: 10.1152/ajprenal.00394.2019. Epub 2019 Sep 18. Am J Physiol Renal Physiol. 2019. PMID: 31532244 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

