Pharmacokinetics of L-Triiodothyronine in Patients Undergoing Thyroid Hormone Therapy Withdrawal
- PMID: 31364488
- PMCID: PMC6797066
- DOI: 10.1089/thy.2019.0101
Pharmacokinetics of L-Triiodothyronine in Patients Undergoing Thyroid Hormone Therapy Withdrawal
Abstract
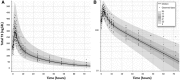
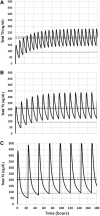
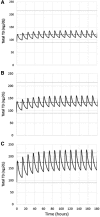
Background: L-triiodothyronine (LT3) is a substitute for levothyroxine (LT4) for thyroid cancer (TC) patients during the preparation for nuclear medicine procedures, and it is used in combination with LT4 in patients who do not respond to the standard treatment for hypothyroidism. This therapy is commonly done by using fixed doses, potentially resulting in supraphysiologic levels of triiodothyronine (T3). A good understanding of the LT3 pharmacokinetics (PK) is necessary to design combination treatment schemes that are able to maintain serum T3 levels within the reference range, but data on the PK of LT3 are conflicting. Here, we present a study designed to characterize the PK of LT3 in patients devoid of endogenous thyroid hormone production, and not receiving LT4 therapy. Methods: We performed an open-label, PK study in patients undergoing thyroid hormone withdrawal in preparation for nuclear medicine procedures for the evaluation and treatment of follicular-derived TC. LT3 was substituted for LT4 at a 1:3 mcg/mcg dosage ratio thrice daily for at least 30 days. PK of the last LT3 dose while at steady state and terminal elimination was assessed over 11 days. Thereafter, a PK study was performed following the nuclear medicine procedure in patients who volunteered for a second study. Results: Fourteen patients age 48.5 ± 16.0 years completed the last dose study and five completed the second PK study. PK analysis indicates a time to maximum serum concentration of 1.8 ± 0.32 hours and two distinct phases of linear elimination, with a fast distribution phase and slow elimination phases with half-lives of 2.3 ± 0.11 hours and 22.9 ± 7.7 hours, supporting a two-compartment model. PK modeling predicts that a twice-daily administration of low-dose LT3 (0.07 mcg/kg twice daily) in combination with LT4 can predictably increase the serum T3 concentration without significant peaks above the reference range. Conclusions: The PK of LT3 is well described by a two-compartment model that assumes elimination only from the sampling compartment, with a rapid distribution phase and a slow elimination phase. This information will contribute to design therapeutic strategies for LT3/LT4 combination therapies directed to maintain stable T3 serum levels.
Keywords: L-triiodothyronine; combination therapy; hypothyroidism; pharmacokinetics; thyroid hormone withdrawal.
Conflict of interest statement
Dr. Celi has received consultant fees from Akrimax, IBSA, and Acella. The Division of Endocrinology Diabetes and Metabolism of Virginia Commonwealth University has received an unrestricted grant from IBSA. None of these entities has been involved in the study.
Figures

References
-
- Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. 2000. The Colorado thyroid disease prevalence study. Arch Intern Med 160:526–534 - PubMed
-
- Vanderpump MP. 2011. The epidemiology of thyroid disease. Br Med Bull 99:39–51 - PubMed
-
- Moon S, Kong SH, Choi HS, Hwangbo Y, Lee MK, Moon JH, Jang HC, Cho NH, Park YJ. 2018. Relation of subclinical hypothyroidism is associated with cardiovascular events and all-cause mortality in adults with high cardiovascular risk. Am J Cardiol 122:571–577 - PubMed
-
- Moon S, Kim MJ, Yu JM, Yoo HJ, Park YJ. 2018. Subclinical hypothyroidism and the risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Thyroid 28:1101–1110 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

