Real-World Use of Do-It-Yourself Artificial Pancreas Systems in Children and Adolescents With Type 1 Diabetes: Online Survey and Analysis of Self-Reported Clinical Outcomes
- PMID: 31364599
- PMCID: PMC6691673
- DOI: 10.2196/14087
Real-World Use of Do-It-Yourself Artificial Pancreas Systems in Children and Adolescents With Type 1 Diabetes: Online Survey and Analysis of Self-Reported Clinical Outcomes
Abstract
Background: Patient-driven initiatives have made uptake of Do-it-Yourself Artificial Pancreas Systems (DIYAPS) increasingly popular among people with diabetes of all ages. Observational studies have shown improvements in glycemic control and quality of life among adults with diabetes. However, there is a lack of research examining outcomes of children and adolescents with DIYAPS in everyday life and their social context.
Objective: This survey assesses the self-reported clinical outcomes of a pediatric population using DIYAPS in the real world.
Methods: An online survey was distributed to caregivers to assess the hemoglobin A1c levels and time in range (TIR) before and after DIYAPS initiation and problems during DIYAPS use.
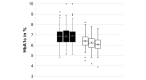
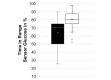
Results: A total of 209 caregivers of children from 21 countries responded to the survey. Of the children, 47.4% were female, with a median age of 10 years, and 99.4% had type 1 diabetes, with a median duration of 4.3 years (SD 3.9). The median duration of DIYAPS use was 7.5 (SD 10.0) months. Clinical outcomes improved significantly, including the hemoglobin A1c levels (from 6.91% [SD 0.88%] to 6.27% [SD 0.67]; P<.001) and TIR (from 64.2% [SD 15.94] to 80.68% [SD 9.26]; P<.001).
Conclusions: Improved glycemic outcomes were found across all pediatric age groups, including adolescents and very young children. These findings are in line with clinical trial results from commercially developed closed-loop systems.
Keywords: artificial pancreas; automated insulin delivery; closed loop; diabetes; do it yourself; mobile health; open source; pediatric diabetes; type 1 diabetes.
©Katarina Braune, Shane O'Donnell, Bryan Cleal, Dana Lewis, Adrian Tappe, Ingrid Willaing, Bastian Hauck, Klemens Raile. Originally published in JMIR Mhealth and Uhealth (http://mhealth.jmir.org), 30.07.2019.
Conflict of interest statement
Conflicts of Interest: KB reports grants from the Berlin Institute of Health (BIH) Clinician Scientist program; fees for medical consulting from Medtronic Diabetes as a member of the Advisory Board "Impact"; medical consulting fees from Roche Diabetes Care; and paid talks for Dexcom, Medtronic Diabetes, and Bertelsmann Stiftung outside the submitted work. AT reports personal fees from Dexcom, Roche Diabetes Care, IME-DC, and Ypsomed; nonfinancial support from Sooil; and personal fees from Gruber-Debong GmbH outside the submitted work. DL reports grants from Robert Wood Johnson Foundation and personal fees from Lilly, Diabeloop, Roche Diabetes Care, Novo Nordisk, and Tandem outside the submitted work. BH reports personal fees from Roche Pharma, Roche Diabetes Care, Novo Nordisk, LifeScan, Bayer AG, and Medtronic Diabetes outside the submitted work. KR is advisory board member of Lilly Diabetes Care and Abbott Diabetes Care outside the submitted work. The remaining authors declare no conflicts of interest.
Figures
References
-
- Diabetes Control and Complications Trial Research Group. Nathan D M, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993 Dec 30;329(14):977–86. doi: 10.1056/NEJM199309303291401. - DOI - PubMed
-
- DiMeglio LA, Acerini CL, Codner E, Craig ME, Hofer SE, Pillay K, Maahs DM. ISPAD Clinical Practice Consensus Guidelines 2018: Glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes. 2018 Dec;19 Suppl 27:105–114. doi: 10.1111/pedi.12737. - DOI - PubMed
-
- Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, Maahs DM, Tamborlane WV, Bergenstal R, Smith E, Olson BA, Garg SK. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther. 2019 Feb;21(2):66–72. doi: 10.1089/dia.2018.0384. - DOI - PMC - PubMed
-
- Tauschmann M, Allen JM, Wilinska ME, Thabit H, Acerini CL, Dunger DB, Hovorka R. Home Use of Day-and-Night Hybrid Closed-Loop Insulin Delivery in Suboptimally Controlled Adolescents With Type 1 Diabetes: A 3-Week, Free-Living, Randomized Crossover Trial. Diabetes Care. 2016 Nov;39(11):2019–2025. doi: 10.2337/dc16-1094. http://europepmc.org/abstract/MED/27612500 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

