Uncovering a Role for the Dorsal Hippocampal Commissure in Recognition Memory
- PMID: 31364703
- PMCID: PMC7132945
- DOI: 10.1093/cercor/bhz143
Uncovering a Role for the Dorsal Hippocampal Commissure in Recognition Memory
Abstract
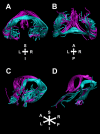
The dorsal hippocampal commissure (DHC) is a white matter tract that provides interhemispheric connections between temporal lobe brain regions. Despite the importance of these regions for learning and memory, there is scant evidence of a role for the DHC in successful memory performance. We used diffusion-weighted magnetic resonance imaging (DW-MRI) and white matter tractography to reconstruct the DHC in both humans (in vivo) and nonhuman primates (ex vivo). Across species, our findings demonstrate a close consistency between the known anatomy and tract reconstructions of the DHC. Anterograde tract-tracer techniques also highlighted the parahippocampal origins of DHC fibers in nonhuman primates. Finally, we derived diffusion tensor MRI metrics from the DHC in a large sample of human subjects to investigate whether interindividual variation in DHC microstructure is predictive of memory performance. The mean diffusivity of the DHC correlated with performance in a standardized recognition memory task, an effect that was not reproduced in a comparison commissure tract-the anterior commissure. These findings highlight a potential role for the DHC in recognition memory, and our tract reconstruction approach has the potential to generate further novel insights into the role of this previously understudied white matter tract in both health and disease.
Keywords: familiarity; hippocampal commissure; recognition memory; recollection; tractography.
© The Author(s) 2019. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures





References
-
- Aggleton JP. 2008. EPS Mid-Career Award 2006. Understanding anterograde amnesia: disconnections and hidden lesions. Q J Exp Psychol (Hove). 61:1441–1471. - PubMed
-
- Aggleton JP. 2012. Multiple anatomical systems embedded within the primate medial temporal lobe: implications for hippocampal function. Neurosci Biobehav Rev. 36:1579–1596. - PubMed
-
- Aggleton JP, Brown MW. 1999. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 22:425–444discussion 444–489. - PubMed
-
- Aggleton JP, Desimone R, Mishkin M. 1986. The origin, course, and termination of the hippocampothalamic projections in the macaque. J Comp Neurol. 243:409–421. - PubMed
-
- Amaral RSC, Park MTM, Devenyi GA, Lynn V, Pipitone J, Winterburn J, Chavez S, Schira M, Lobaugh NJ, Voineskos AN et al. . 2018. Manual segmentation of the fornix, fimbria, and alveus on high-resolution 3T MRI: application via fully-automated mapping of the human memory circuit white and grey matter in healthy and pathological aging. Neuroimage. 170:132–150. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

