Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease
- PMID: 31364924
- PMCID: PMC6890986
- DOI: 10.1152/physrev.00022.2018
Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease
Abstract
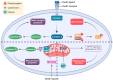
Twelve regulated cell death programs have been described. We review in detail the basic biology of nine including death receptor-mediated apoptosis, death receptor-mediated necrosis (necroptosis), mitochondrial-mediated apoptosis, mitochondrial-mediated necrosis, autophagy-dependent cell death, ferroptosis, pyroptosis, parthanatos, and immunogenic cell death. This is followed by a dissection of the roles of these cell death programs in the major cardiac syndromes: myocardial infarction and heart failure. The most important conclusion relevant to heart disease is that regulated forms of cardiomyocyte death play important roles in both myocardial infarction with reperfusion (ischemia/reperfusion) and heart failure. While a role for apoptosis in ischemia/reperfusion cannot be excluded, regulated forms of necrosis, through both death receptor and mitochondrial pathways, are critical. Ferroptosis and parthanatos are also likely important in ischemia/reperfusion, although it is unclear if these entities are functioning as independent death programs or as amplification mechanisms for necrotic cell death. Pyroptosis may also contribute to ischemia/reperfusion injury, but potentially through effects in non-cardiomyocytes. Cardiomyocyte loss through apoptosis and necrosis is also an important component in the pathogenesis of heart failure and is mediated by both death receptor and mitochondrial signaling. Roles for immunogenic cell death in cardiac disease remain to be defined but merit study in this era of immune checkpoint cancer therapy. Biology-based approaches to inhibit cell death in the various cardiac syndromes are also discussed.
Keywords: apoptosis; cell death; heart disease; necroptosis; necrosis.
Conflict of interest statement
A. Linkermann has received research grants from Pfizer, Novartis, Fresenius Medical Care, and the Else Kröner-Fresenius Stiftung; has material transfer agreements with Genentech, Glaxo Smith Kline, and Apogenix; and has received honoraria and/or travel grants from Astellas, Otsuka, Genentech, Alexion, and Tekmira. R.N. Kitsis is co-founder of Aspida Therapeutics Inc. and a consultant for Amaron Bio. No conflicts of interest, financial or otherwise, are declared by the other authors.
Figures







References
-
- Abelson S, Collord G, Ng SWK, Weissbrod O, Mendelson Cohen N, Niemeyer E, Barda N, Zuzarte PC, Heisler L, Sundaravadanam Y, Luben R, Hayat S, Wang TT, Zhao Z, Cirlan I, Pugh TJ, Soave D, Ng K, Latimer C, Hardy C, Raine K, Jones D, Hoult D, Britten A, McPherson JD, Johansson M, Mbabaali F, Eagles J, Miller JK, Pasternack D, Timms L, Krzyzanowski P, Awadalla P, Costa R, Segal E, Bratman SV, Beer P, Behjati S, Martincorena I, Wang JCY, Bowles KM, Quirós JR, Karakatsani A, La Vecchia C, Trichopoulou A, Salamanca-Fernández E, Huerta JM, Barricarte A, Travis RC, Tumino R, Masala G, Boeing H, Panico S, Kaaks R, Krämer A, Sieri S, Riboli E, Vineis P, Foll M, McKay J, Polidoro S, Sala N, Khaw K-T, Vermeulen R, Campbell PJ, Papaemmanuil E, Minden MD, Tanay A, Balicer RD, Wareham NJ, Gerstung M, Dick JE, Brennan P, Vassiliou GS, Shlush LI. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature 559: 400–404, 2018. doi: 10.1038/s41586-018-0317-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

