Simplify: A Mass Spectrometry Metabolomics Approach to Identify Additives and Synergists from Complex Mixtures
- PMID: 31365233
- PMCID: PMC6817979
- DOI: 10.1021/acs.analchem.9b02377
Simplify: A Mass Spectrometry Metabolomics Approach to Identify Additives and Synergists from Complex Mixtures
Abstract
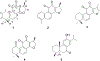
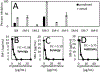
In fields ranging from environmental toxicology to drug discovery, it is critical to identify how multiple chemical compounds interact to perturb biological systems. Isolation-based approaches fail to incorporate multiconstituent interactions, such as synergy. We have developed an approach called "Simplify", which identifies mixture constituents that interact to achieve biological effects. Simplify combines biological and mass spectrometric data sets and uses an "activity index" to predict mixture interactions. Using the plant Salvia miltiorrhiza as a case study, we employed Simplify to identify four individual constituents that contribute to antimicrobial activity, three additives and one synergist. Our study is the first to enable identification of unknown synergists prior to isolating them, demonstrating the ability of the Simplify workflow to predict key contributors to the biological effect of a complex mixture. While utilized for natural products discovery in this study, this approach is expected to prove useful across multiple disciplines that rely on mixture analysis.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
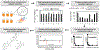
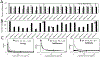


References
-
- Health Effects Institute. In Theoretical Approaches to Analyzing Complex Mixtures; Heath Effects Institute: Cambridge, MA, 1996.
-
- Snyder SA J. Am. Water Works 2014, 106, 38–52.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

