Downregulation of calponin 2 contributes to the quiescence of lung macrophages
- PMID: 31365293
- PMCID: PMC6850996
- DOI: 10.1152/ajpcell.00036.2019
Downregulation of calponin 2 contributes to the quiescence of lung macrophages
Abstract
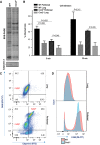
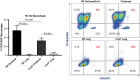
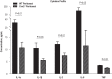
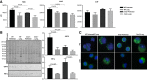
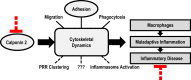
Calponin 2 is an actin cytoskeleton-associated regulatory protein that inhibits the activity of myosin-ATPase and cytoskeleton dynamics. Recent studies have demonstrated that deletion of calponin 2 restricts the proinflammatory activation of macrophages in atherosclerosis and arthritis to attenuate the disease progression in mice. Here we demonstrate that the levels of calponin 2 vary among different macrophage populations, which may reflect their adaptation to specific tissue microenvironment corresponding to specific functional states. Interestingly, lung resident macrophages express significantly lower calponin 2 than peritoneal resident macrophages, which correlates with decreased substrate adhesion and reduced expression of proinflammatory cytokines and a proresolution phenotype. Deletion of calponin 2 in peritoneal macrophages also decreased substrate adhesion and downregulated the expression of proinflammatory cytokines. Providing the first line of defense against microbial invasion while receiving constant exposure to extrinsic antigens, lung macrophages need to maintain a necessary level of activity while limiting exaggerated inflammatory reaction. Therefore, their low level of calponin 2 may reflect an important physiological adaption. Downregulation of calponin 2 in macrophages may be targeted as a cytoskeleton-based novel mechanism, possibly via endoplasmic reticulum stress altering the processing and secretion of cytokines, to regulate immune response and promote quiescence for the treatment of inflammatory diseases.
Keywords: actin; adhesion; cytoskeleton; inflammation; macrophage.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



References
-
- Bain CC, Hawley CA, Garner H, Scott CL, Schridde A, Steers NJ, Mack M, Joshi A, Guilliams M, Mowat AM, Geissmann F, Jenkins SJ. Long-lived self-renewing bone marrow-derived macrophages displace embryo-derived cells to inhabit adult serous cavities. Nat Commun 7: ncomms11852, 2016. doi: 10.1038/ncomms11852. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

