Impaired glucose tolerance, glucagon, and insulin responses in mice lacking the loop diuretic-sensitive Nkcc2a transporter
- PMID: 31365295
- PMCID: PMC6850998
- DOI: 10.1152/ajpcell.00144.2019
Impaired glucose tolerance, glucagon, and insulin responses in mice lacking the loop diuretic-sensitive Nkcc2a transporter
Abstract
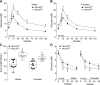
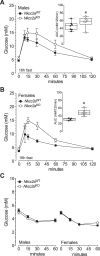
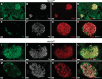
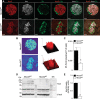
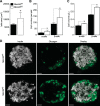
The Na+K+2Cl- cotransporter-2 (Nkcc2, Slc12a1) is abundantly expressed in the kidney and its inhibition with the loop-diuretics bumetanide and furosemide has been linked to transient or permanent hyperglycemia in mice and humans. Notably, Slc12a1 is expressed at low levels in hypothalamic neurons and in insulin-secreting β-cells of the endocrine pancreas. The present study was designed to determine if global elimination of one of the Slc12a1 products, i.e., Nkcc2 variant a (Nkcc2a), the main splice version of Nkcc2 found in insulin-secreting β-cells, has an impact on the insulin and glucagon secretory responses and fuel homeostasis in vivo. We have used dynamic tests of glucose homeostasis in wild-type mice and mice lacking both alleles of Nkcc2a (Nkcc2aKO) and assessed their islet secretory responses in vitro. Under basal conditions, Nkcc2aKO mice have impaired glucose homeostasis characterized by increased blood glucose, intolerance to the sugar, delayed/blunted in vivo insulin and glucagon responses to glucose, and increased glycemic responses to the gluconeogenic substrate alanine. Further, we provide evidence of conserved quantitative secretory responses of Nkcc2aKO islets within a context of increased islet size related to hyperplastic/hypertrophic glucagon- and insulin-positive cells (α-cells and β-cells, respectively), normal total islet Cl- content, and reduced β-cell expression of the Cl- extruder Kcc2.
Keywords: Slc12a1/Nkcc2a; Slc12a5/Kcc2; glucagon; glucose homeostasis; insulin.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


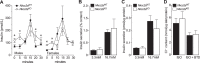

References
-
- Abramoff MD, Magalhães PJ, Ram SJ. Image processing using ImageJ. Biophoton Int 11: 36–42, 2004.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

