Beta-band motor unit coherence and nonlinear surface EMG features of the first dorsal interosseous muscle vary with force
- PMID: 31365308
- PMCID: PMC6766730
- DOI: 10.1152/jn.00228.2019
Beta-band motor unit coherence and nonlinear surface EMG features of the first dorsal interosseous muscle vary with force
Abstract
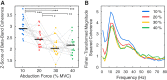
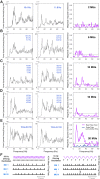
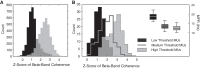
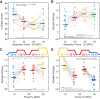
Motor unit firing times are weakly coupled across a range of frequencies during voluntary contractions. Coherent activity within the beta-band (15-35 Hz) has been linked to oscillatory cortical processes, providing evidence of functional connectivity between the motoneuron pool and motor cortex. The aim of this study was to investigate whether beta-band motor unit coherence is altered with increasing abduction force in the first dorsal interosseous muscle. Coherence between motor unit firing times, extracted from decomposed surface electromyography (EMG) signals, was investigated in 17 subjects at 10, 20, 30, and 40% of maximum voluntary contraction. Corresponding changes in nonlinear surface EMG features (specifically sample entropy and determinism, which are sensitive to motor unit synchronization) were also examined. A reduction in beta-band and alpha-band coherence was observed as force increased [F(3, 151) = 32, P < 0.001 and F(3, 151) = 27, P < 0.001, respectively], accompanied by corresponding changes in nonlinear surface EMG features. A significant relationship between the nonlinear features and motor unit coherence was also detected (r = -0.43 ± 0.1 and r = 0.45 ± 0.1 for sample entropy and determinism, respectively; both P < 0.001). The reduction in beta-band coherence suggests a change in the relative contribution of correlated and uncorrelated presynaptic inputs to the motoneuron pool, and/or a decrease in the responsiveness of the motoneuron pool to synchronous inputs at higher forces. The study highlights the importance of considering muscle activation when investigating changes in motor unit coherence or nonlinear EMG features and examines other factors that can influence coherence estimation.NEW & NOTEWORTHY Intramuscular alpha- and beta-band coherence decreased as muscle contraction force increased. Beta-band coherence was higher in groups of high-threshold motor units than in simultaneously active lower threshold units. Alterations in motor unit coherence with increases or decreases in force and with the onset of fatigue were accompanied by corresponding changes in surface electromyography sample entropy and determinism. Mixed-model analysis indicated mean firing rate and number of motor units also influenced the coherence estimate.
Keywords: EMG; coherence; determinism; motor unit; sample entropy.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

