Acceptance of Opt-Out HIV Screening in Outpatient Settings in the United States: A Systematic Review and Meta-Analysis
- PMID: 31365316
- PMCID: PMC6852056
- DOI: 10.1177/0033354919860510
Acceptance of Opt-Out HIV Screening in Outpatient Settings in the United States: A Systematic Review and Meta-Analysis
Abstract
Objectives: In the United States, about 15% of persons living with HIV infection do not know they are infected. Opt-out HIV screening aims to normalize HIV testing by performing an HIV test during routine medical care unless the patient declines. The primary objective of this systematic review and meta-analysis was to assess the acceptance of opt-out HIV screening in outpatient settings in the United States.
Methods: We searched in PubMed and CINAHL (Cumulative Index to Nursing and Allied Health Literature) for studies published from January 1, 2006, through December 31, 2018, of opt-out HIV screening in outpatient settings. We collected data from selected studies and calculated for each study (1) the percentage of persons who were offered HIV testing, (2) the percentage of persons who accepted the test, and (3) the percentage of new HIV diagnoses among persons tested. We also collected information on the reasons given by patients for opting out. The meta-analysis used a random-effects model to estimate the average percentages of HIV testing offered, HIV testing accepted, and new HIV diagnoses.
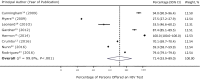
Results: We initially identified 6986 studies; the final analysis comprised 14 studies. Among the 8 studies that reported the size of the study population eligible for HIV screening, 71.4% (95% confidence interval [CI], 53.9%-89.0%) of the population was offered an HIV test on an opt-out basis. The test was accepted by 58.7% (95% CI, 47.2%-70.2%) of persons offered the test. Among 9 studies that reported data on new HIV diagnoses, 0.18% (95% CI, 0.08%-0.26%) of the persons tested had a new HIV diagnosis. Patients' most frequently cited reasons for refusal of HIV screening were that they perceived a low risk of having HIV or had previously been tested.
Conclusions: The rates of offering and accepting an HIV test on an opt-out basis could be improved by addressing health system and patient-related factors. Setting a working target for these rates would be useful for measuring the success of opt-out HIV screening programs.
Keywords: HIV; opt-out; outpatient; routine; screening.
Conflict of interest statement
Figures


References
-
- Centers for Disease Control and Prevention. HIV/AIDS. Basic statistics. 2018. https://www.cdc.gov/hiv/basics/statistics.html. Accessed February 2, 2019.
-
- Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. - PubMed
-
- Maricopa Integrated Health System. What is opt-out testing? https://www.mihs.org/testaz/benefits-of-hiv-testing/what-is-opt-out-testing. Accessed January 29, 2019.
-
- US Preventive Services Task Force. USPSTF A and B recommendations. 2017. https://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-r.... Accessed February 5, 2019.
-
- Qaseem A, Snow V, Shekelle P, Hopkins R, Jr, Owens DK; Clinical Efficacy Assessment Subcommittee, American College of Physicians. Screening for HIV in health care settings: a guidance statement from the American College of Physicians and HIV Medicine Association. Ann Intern Med. 2009;150(2):125–131. doi:10.7326/0003-4819-150-2-200901200-00300 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

