Changes in Hepatic Venous Pressure Gradient Predict Hepatic Decompensation in Patients Who Achieved Sustained Virologic Response to Interferon-Free Therapy
- PMID: 31365764
- PMCID: PMC7155089
- DOI: 10.1002/hep.30885
Changes in Hepatic Venous Pressure Gradient Predict Hepatic Decompensation in Patients Who Achieved Sustained Virologic Response to Interferon-Free Therapy
Abstract
Background and aims: Sustained virologic response (SVR) to interferon (IFN)-free therapies ameliorates portal hypertension (PH); however, it remains unclear whether a decrease in hepatic venous pressure gradient (HVPG) after cure of hepatitis C translates into a clinical benefit. We assessed the impact of pretreatment HVPG, changes in HVPG, and posttreatment HVPG on the development of hepatic decompensation in patients with PH who achieved SVR to IFN-free therapy. Moreover, we evaluated transient elastography (TE) and von Willebrand factor to platelet count ratio (VITRO) as noninvasive methods for monitoring the evolution of PH.
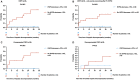
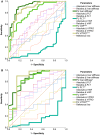
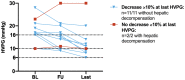
Approach and results: The study comprised 90 patients with HVPG ≥ 6 mm Hg who underwent paired HVPG, TE, and VITRO assessments before (baseline [BL]) and after (follow-up [FU]) IFN-free therapy. FU HVPG but not BL HVPG predicted hepatic decompensation (per mm Hg, hazard ratio, 1.18; 95% confidence interval, 1.08-1.28; P < 0.001). Patients with BL HVPG ≤ 9 mm Hg or patients who resolved clinically significant PH (CSPH) were protected from hepatic decompensation. In patients with CSPH, an HVPG decrease ≥ 10% was similarly protective (36 months, 2.5% vs. 40.5%; P < 0.001) but was observed in a substantially higher proportion of patients (60% vs. 24%; P < 0.001). Importantly, the performance of noninvasive methods such as TE/VITRO for diagnosing an HVPG reduction ≥ 10% was inadequate for clinical use (area under the receiver operating characteristic curve [AUROC], < 0.8), emphasizing the need for HVPG measurements. However, TE/VITRO were able to rule in or rule out FU CSPH (AUROC, 0.86-0.92) in most patients, especially if assessed in a sequential manner.
Conclusions: Reassessment of HVPG after SVR improved prognostication in patients with pretreatment CSPH. An "immediate" HVPG decrease ≥ 10% was observed in the majority of these patients and was associated with a clinical benefit, as it prevented hepatic decompensation. These results support the use of HVPG as a surrogate endpoint for interventions that lower portal pressure by decreasing intrahepatic resistance.
© 2019 The Authors. Hepatology published by Wiley Periodicals, Inc., on behalf of American Association for the Study of Liver Diseases.
Figures

References
-
- Mandorfer M, Schwabl P, Steiner S, Scheiner B, Chromy D, Bucsics T, et al. Interferon‐free treatment with sofosbuvir/daclatasvir achieves sustained virologic response in 100% of HIV/hepatitis C virus‐coinfected patients with advanced liver disease. AIDS 2016;30:1039‐1047. - PubMed
-
- Mandorfer M, Schwabl P, Steiner S, Reiberger T, Peck‐Radosavljevic M. Advances in the management of HIV/HCV coinfection. Hepatol Int 2016;10:424‐435. - PubMed
-
- Mandorfer M, Kozbial K, Freissmuth C, Schwabl P, Stattermayer AF, Reiberger T, et al. Interferon‐free regimens for chronic hepatitis C overcome the effects of portal hypertension on virological responses. Aliment Pharmacol Ther 2015;42:707‐718. - PubMed
-
- Ferenci P, Kozbial K, Mandorfer M, Hofer H. HCV targeting of patients with cirrhosis. J Hepatol 2015;63:1015‐1022. - PubMed
-
- Jacobson IM, Lim JK, Fried MW. American Gastroenterological Association Institute Clinical Practice Update‐Expert Review: care of patients who have achieved a sustained virologic response after antiviral therapy for chronic hepatitis C infection. Gastroenterology 2017;152:1578‐1587. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

