Ibrutinib-Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia
- PMID: 31365801
- PMCID: PMC6908306
- DOI: 10.1056/NEJMoa1817073
Ibrutinib-Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia
Abstract
Background: Data regarding the efficacy of treatment with ibrutinib-rituximab, as compared with standard chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab, in patients with previously untreated chronic lymphocytic leukemia (CLL) have been limited.
Methods: In a phase 3 trial, we randomly assigned (in a 2:1 ratio) patients 70 years of age or younger with previously untreated CLL to receive either ibrutinib and rituximab for six cycles (after a single cycle of ibrutinib alone), followed by ibrutinib until disease progression, or six cycles of chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab. The primary end point was progression-free survival, and overall survival was a secondary end point. We report the results of a planned interim analysis.
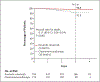
Results: A total of 529 patients underwent randomization (354 patients to the ibrutinib-rituximab group, and 175 to the chemoimmunotherapy group). At a median follow-up of 33.6 months, the results of the analysis of progression-free survival favored ibrutinib-rituximab over chemoimmunotherapy (89.4% vs. 72.9% at 3 years; hazard ratio for progression or death, 0.35; 95% confidence interval [CI], 0.22 to 0.56; P<0.001), and the results met the protocol-defined efficacy threshold for the interim analysis. The results of the analysis of overall survival also favored ibrutinib-rituximab over chemoimmunotherapy (98.8% vs. 91.5% at 3 years; hazard ratio for death, 0.17; 95% CI, 0.05 to 0.54; P<0.001). In a subgroup analysis involving patients without immunoglobulin heavy-chain variable region (IGHV) mutation, ibrutinib-rituximab resulted in better progression-free survival than chemoimmunotherapy (90.7% vs. 62.5% at 3 years; hazard ratio for progression or death, 0.26; 95% CI, 0.14 to 0.50). The 3-year progression-free survival among patients with IGHV mutation was 87.7% in the ibrutinib-rituximab group and 88.0% in the chemoimmunotherapy group (hazard ratio for progression or death, 0.44; 95% CI, 0.14 to 1.36). The incidence of adverse events of grade 3 or higher (regardless of attribution) was similar in the two groups (in 282 of 352 patients [80.1%] who received ibrutinib-rituximab and in 126 of 158 [79.7%] who received chemoimmunotherapy), whereas infectious complications of grade 3 or higher were less common with ibrutinib-rituximab than with chemoimmunotherapy (in 37 patients [10.5%] vs. 32 [20.3%], P<0.001).
Conclusions: The ibrutinib-rituximab regimen resulted in progression-free survival and overall survival that were superior to those with a standard chemoimmunotherapy regimen among patients 70 years of age or younger with previously untreated CLL. (Funded by the National Cancer Institute and Pharmacyclics; E1912 ClinicalTrials.gov number, NCT02048813.).
Copyright © 2019 Massachusetts Medical Society.
Figures


Comment in
-
Ibrutinib outperforms FCR in CLL.Nat Rev Clin Oncol. 2019 Oct;16(10):592. doi: 10.1038/s41571-019-0265-6. Nat Rev Clin Oncol. 2019. PMID: 31417194 No abstract available.
References
-
- Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010; 376: 1164–74. - PubMed
-
- Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014; 370: 1101–10. - PubMed
-
- Eichhorst B, Fink AM, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol 2016; 17: 928–42. - PubMed
-
- Stilgenbauer S, Schnaiter A, Paschka P, et al. Gene mutations and treatment outcome in chronic lymphocytic leukemia: results from the CLL8 trial. Blood 2014; 123: 3247–54. - PubMed
-
- Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood 2016; 127: 208–15. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UG1 CA233290/CA/NCI NIH HHS/United States
- UG1 CA189821/CA/NCI NIH HHS/United States
- CA180855/CA/NCI NIH HHS/United States
- UG1 CA233330/CA/NCI NIH HHS/United States
- U24 CA196172/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- CA180820/CA/NCI NIH HHS/United States
- UG1 CA189828/CA/NCI NIH HHS/United States
- CA180790/CA/NCI NIH HHS/United States
- P30 CA014236/CA/NCI NIH HHS/United States
- U10 CA180855/CA/NCI NIH HHS/United States
- U10 CA180867/CA/NCI NIH HHS/United States
- U10 CA180816/CA/NCI NIH HHS/United States
- UG1 CA233339/CA/NCI NIH HHS/United States
- CA190140/CA/NCI NIH HHS/United States
- R01 CA193541/CA/NCI NIH HHS/United States
- CA180833/CA/NCI NIH HHS/United States
- CA180888/CA/NCI NIH HHS/United States
- UG1 CA190140/CA/NCI NIH HHS/United States
- CA193541/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U10 CA180791/CA/NCI NIH HHS/United States
- CA189863/CA/NCI NIH HHS/United States
- UG1 CA189863/CA/NCI NIH HHS/United States
- CA180816/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States
- CA180794/CA/NCI NIH HHS/United States
- U10 CA180790/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180833/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- CA189821/CA/NCI NIH HHS/United States
- CA180791/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- CA180821/CA/NCI NIH HHS/United States
- CA180867/CA/NCI NIH HHS/United States
- CA189828/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
