Transactivation Function-1-Mediated Partial Agonist Activity of Selective Estrogen Receptor Modulator Requires Homo-Dimerization of the Estrogen Receptor α Ligand Binding Domain
- PMID: 31366023
- PMCID: PMC6695978
- DOI: 10.3390/ijms20153718
Transactivation Function-1-Mediated Partial Agonist Activity of Selective Estrogen Receptor Modulator Requires Homo-Dimerization of the Estrogen Receptor α Ligand Binding Domain
Abstract
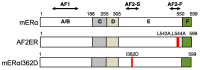
The isolation of estrogen receptor alpha (ERα) cDNA was successful around 30 years ago. The characteristics of ERα protein have been examined from various aspects, primarily through in vitro cell culture studies, but more recently using in vivo experimental models. There remains, however, some uncharacterized ERα functionalities. In particular, the mechanism of partial agonist activity of selective estrogen receptor modulators (SERMs) that involves control of the N-terminal transcription function of ERα, termed AF-1, is still an unsolved ERα functionality. We review the possible mechanism of SERM-dependent regulation of ERα AF-1-mediated transcriptional activity, which includes the role of helix 12 of ERα ligand binding domain (LBD) for SERM-dependent AF-1 regulation. In addition, we describe a specific portion of the LBD that associates with blocking AF-1 activity with an additional role of the F-domain in mediating SERM activity.
Keywords: AF-1; F-domain; estrogen receptor alpha; homo-dimerization; ligand binding domain; selective estrogen receptor modulator.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
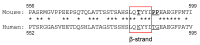
Similar articles
-
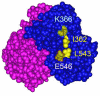
Estrogen receptor α L543A,L544A mutation changes antagonists to agonists, correlating with the ligand binding domain dimerization associated with DNA binding activity.J Biol Chem. 2013 Jul 19;288(29):21105-21116. doi: 10.1074/jbc.M113.463455. Epub 2013 Jun 3. J Biol Chem. 2013. PMID: 23733188 Free PMC article.
-
The physiological role of estrogen receptor functional domains.Essays Biochem. 2021 Dec 17;65(6):867-875. doi: 10.1042/EBC20200167. Essays Biochem. 2021. PMID: 34028522 Free PMC article. Review.
-
The F domain of estrogen receptor α is involved in species-specific, tamoxifen-mediated transactivation.J Biol Chem. 2018 Jun 1;293(22):8495-8507. doi: 10.1074/jbc.RA117.001212. Epub 2018 Apr 9. J Biol Chem. 2018. PMID: 29632071 Free PMC article.
-
Transactivation Function-2 of Estrogen Receptor α Contains Transactivation Function-1-regulating Element.J Biol Chem. 2015 Jul 10;290(28):17611-27. doi: 10.1074/jbc.M115.638650. Epub 2015 May 31. J Biol Chem. 2015. PMID: 26028650 Free PMC article.
-
Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology.J Steroid Biochem Mol Biol. 2000 Nov 30;74(5):279-85. doi: 10.1016/s0960-0760(00)00104-7. J Steroid Biochem Mol Biol. 2000. PMID: 11162936 Review.
Cited by
-
Roles of estrogen receptor α in endometrial carcinoma (Review).Oncol Lett. 2023 Oct 25;26(6):530. doi: 10.3892/ol.2023.14117. eCollection 2023 Dec. Oncol Lett. 2023. PMID: 38020303 Free PMC article. Review.
-
Roles of Estrogen, Estrogen Receptors, and Estrogen-Related Receptors in Skeletal Muscle: Regulation of Mitochondrial Function.Int J Mol Sci. 2023 Jan 17;24(3):1853. doi: 10.3390/ijms24031853. Int J Mol Sci. 2023. PMID: 36768177 Free PMC article. Review.
-
Endocrine Therapy-related Endocrinopathies-Biology, Prevalence and Implications for the Management of Breast Cancer.Oncol Hematol Rev. 2020 Spring;16(1):17-22. doi: 10.17925/ohr.2020.16.1.17. Epub 2020 Feb 16. Oncol Hematol Rev. 2020. PMID: 33841882 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

