HIV Viral Load Estimation Using Hematocrit Corrected Dried Blood Spot Results on a BioMerieux NucliSENS® Platform
- PMID: 31366024
- PMCID: PMC6787611
- DOI: 10.3390/diagnostics9030086
HIV Viral Load Estimation Using Hematocrit Corrected Dried Blood Spot Results on a BioMerieux NucliSENS® Platform
Abstract
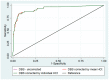
While reporting human immunodeficiency virus (HIV) viral load (VL) using dried blood spot (DBS) in the BioMerieux NucliSENS platform, application of the hematocrit correction factor has been suggested. In this cross-sectional study from the National Microbiology Reference Laboratory of Zimbabwe, we assessed whether hematocrit correction (individual and/or mean) in DBS results improved the correlation with plasma VL and prediction of VL non-suppression (≥1000 copies per ml in plasma). Of 517 specimens during August-December 2018, 65(12.6%) had non-suppressed plasma VL results. The hematocrit correction factor ranged from 1.3 to 2.0 with a mean of 1.6, standard deviation (SD: 1.5, 1.7). The intraclass correlation (ICC) for mean (0.859, 95% CI: 0.834, 0.880) and individual (0.809, 95% CI: 0.777, 0.837) hematocrit corrected DBS results were not significantly different. The uncorrected DBS results had a significantly lower ICC (0.640, 95% CI: 0.586, 0.688) when compared to corrected DBS results. There were no significant differences in validity, predictive values, and areas under the receiver operating characteristics curves for all three DBS results when predicting VL non-suppression. To conclude, hematocrit correction of DBS VL results improved agreement with the plasma results but did not improve prediction of VL non-suppression. The results were not significantly different for individual and mean corrected results.
Keywords: Antiretroviral therapy (ART) monitoring; dried blood spot; hematocrit; human immunodeficiency virus; viral load.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Performance of patient-collected dried blood specimens for HIV-1 viral load testing in South Africa.AIDS. 2024 Dec 1;38(15):2050-2055. doi: 10.1097/QAD.0000000000004011. Epub 2024 Sep 12. AIDS. 2024. PMID: 39264578 Free PMC article. Clinical Trial.
-
Performance of a Novel Low-Cost, Instrument-Free Plasma Separation Device for HIV Viral Load Quantification and Determination of Treatment Failure in People Living with HIV in Malaysia: a Diagnostic Accuracy Study.J Clin Microbiol. 2019 Mar 28;57(4):e01683-18. doi: 10.1128/JCM.01683-18. Print 2019 Apr. J Clin Microbiol. 2019. PMID: 30700508 Free PMC article.
-
Finger Prick Dried Blood Spots for HIV Viral Load Measurement in Field Conditions in Zimbabwe.PLoS One. 2015 May 22;10(5):e0126878. doi: 10.1371/journal.pone.0126878. eCollection 2015. PLoS One. 2015. PMID: 26001044 Free PMC article.
-
Accurate HIV viral load measurement in primary health care settings using the cobas® plasma separation card.PLoS One. 2020 May 6;15(5):e0232122. doi: 10.1371/journal.pone.0232122. eCollection 2020. PLoS One. 2020. PMID: 32374748 Free PMC article.
-
Sensitivity and specificity of two dried blood spot methods for HIV-1 viral load monitoring among patients in Hanoi, Vietnam.PLoS One. 2018 Jan 18;13(1):e0191411. doi: 10.1371/journal.pone.0191411. eCollection 2018. PLoS One. 2018. PMID: 29346431 Free PMC article.
Cited by
-
HIV RNA measurement in dried blood spots of HIV-infected patients in Thailand using Abbott m2000 system.PLoS One. 2020 Jan 24;15(1):e0227929. doi: 10.1371/journal.pone.0227929. eCollection 2020. PLoS One. 2020. PMID: 31978113 Free PMC article.
-
Whatman FTA cards versus plasma specimens for the quantitation of HIV-1 RNA using two real-time PCR assays.Access Microbiol. 2020 Jun 17;2(8):acmi000138. doi: 10.1099/acmi.0.000138. eCollection 2020. Access Microbiol. 2020. PMID: 32974600 Free PMC article.
References
-
- UNAIDS . 90-90-90 An Ambitious Treatment Target to Help End the AIDS Epidemic. UNAIDS; Geneva, Switzerland: 2014.
-
- World Health Organization (WHO) Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. WHO; Geneva, Switzerland: 2016. - PubMed
-
- World Health Organization (WHO) Technical and Operational Considerations for Implementing HIV Viral Load Testing: Interim Technical Update. WHO; Geneva, Switzerland: 2014.
LinkOut - more resources
Full Text Sources

