Interspecies Variation in NCMN -O- Demethylation in Liver Microsomes from Various Species
- PMID: 31366067
- PMCID: PMC6695839
- DOI: 10.3390/molecules24152765
Interspecies Variation in NCMN -O- Demethylation in Liver Microsomes from Various Species
Abstract
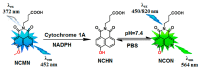
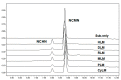
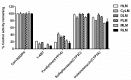
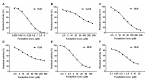
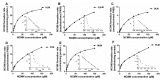
NCMN (N-(3-carboxy propyl)-4-methoxy-1,8-naphthalimide), a newly developed ratiometric two-photon fluorescent probe for human Cytochrome P450 1A (CYP1A), shows the best combination of specificity and reactivity for real-time detection of the enzymatic activities of CYP1A in complex biological systems. This study aimed to investigate the interspecies variation in NCMN-O-demethylation in commercially available liver microsomes from human, mouse, rat, beagle dog, minipig and cynomolgus monkey. Metabolite profiling demonstrated that NCMN could be O-demethylated in liver microsomes from all species but the reaction rate varied considerably. CYP1A was the major isoform involved in NCMN-O-demethylation in all examined liver microsomes based on the chemical inhibition assays. Furafylline, a specific inhibitor of mammalian CYP1A, displayed differential inhibitory effects on NCMN-O-demethylation in all tested species. Kinetic analyses demonstrated that NCMN-O-demethylation in liver microsomes form rat, minipig and cynomolgus monkey followed biphasic kinetics, while in liver microsomes form human, mouse and beagle dog obeyed Michaelis-Menten kinetics, the kinetic parameters from various species are much varied, while NCMN-O-demethylation in MLM exhibited the highest similarity of specificity, kinetic behavior and intrinsic clearance as that in HLM. These findings will be very helpful for the rational use of NCMN as a practical tool to decipher the functions of mammalian CYP1A or to study CYP1A associated drug-drug interactions in vivo.
Keywords: Cytochrome P450 1A (CYP1A); NCMN-O-demethylation; liver microsomes; species differences.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A Highly Selective Ratiometric Two-Photon Fluorescent Probe for Human Cytochrome P450 1A.J Am Chem Soc. 2015 Nov 18;137(45):14488-95. doi: 10.1021/jacs.5b09854. Epub 2015 Nov 4. J Am Chem Soc. 2015. PMID: 26488456
-
A comparative study of constitutive and induced alkoxyresorufin O-dealkylation and individual cytochrome P450 forms in cynomolgus monkey (Macaca fascicularis), human, mouse, rat and hamster liver microsomes.Biochem Pharmacol. 1994 Mar 2;47(5):763-73. doi: 10.1016/0006-2952(94)90475-8. Biochem Pharmacol. 1994. PMID: 8135852
-
Cytochrome P450 specificities of alkoxyresorufin O-dealkylation in human and rat liver.Biochem Pharmacol. 1994 Aug 30;48(5):923-36. doi: 10.1016/0006-2952(94)90363-8. Biochem Pharmacol. 1994. PMID: 8093105
-
CYP1A-mediated metabolism of the Janus kinase-3 inhibitor 4-(4'-hydroxyphenyl)-amino-6,7-dimethoxyquinazoline: structural basis for inactivation by regioselective O-demethylation.Drug Metab Dispos. 2002 Jan;30(1):74-85. doi: 10.1124/dmd.30.1.74. Drug Metab Dispos. 2002. PMID: 11744615
-
Cytochrome P450-mediated hepatic metabolism of new fluorescent substrates in cats and dogs.J Vet Pharmacol Ther. 2010 Dec;33(6):519-27. doi: 10.1111/j.1365-2885.2010.01199.x. J Vet Pharmacol Ther. 2010. PMID: 21062303 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

