Dental Implants with Anti-Biofilm Properties: A Pilot Study for Developing a New Sericin-Based Coating
- PMID: 31366076
- PMCID: PMC6695694
- DOI: 10.3390/ma12152429
Dental Implants with Anti-Biofilm Properties: A Pilot Study for Developing a New Sericin-Based Coating
Abstract
Aim: several strategies have been tested in recent years to prevent bacterial colonization of dental implants. Sericin, one of the two main silk proteins, possesses relevant biological activities and also literature reports about its potential antibacterial properties, but results are discordant and not yet definitive. The aim of this study was to evaluate the effectiveness of different experimental protocols in order to obtain a sericin-based coating on medical grade titanium (Ti) able to reduce microbial adhesion to the dental implant surface.
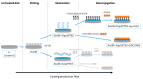
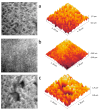
Materials and methods: different strategies for covalent bonding of sericin to Ti were pursued throughout a multi-step procedure on Ti-6Al-4V disks. The surface of grade 5 Ti was initially immersed in NaOH solution to obtain the exposure of functional -OH groups. Two different silanization strategies were then tested using aminopropyltriethoxysilane (APTES). Eventually, the bonding between silanized Ti-6Al-4V and sericin was obtained with two different crosslinking processes: glutaraldehyde (GLU) or carbodiimide/N-Hydroxy-succinimide (EDC/NHS). Micro-morphological and compositional analyses were performed on the samples at each intermediate step to assess the most effective coating strategy able to optimize the silanization and bioconjugation processes. Microbiological tests on the coated Ti-6Al-4V disks were conducted in vitro using a standard biofilm producer strain of Staphylococcus aureus (ATCC 6538) to quantify the inhibition of microbial biofilm formation (anti-biofilm efficacy) at 24 hours.
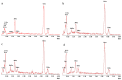
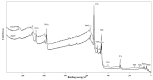
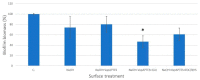
Results: both silanization techniques resulted in a significant increase of silicon (Si) on the Ti-6Al-4V surfaces etched with NaOH. Differences were found between GLU and EDC/NHS bioconjugation strategies in terms of composition, surface micro-morphology and anti-biofilm efficacy. Ti-6Al-4V samples coated with GLU-bound sericin after silanization obtained via vapor phase deposition proved that this technique is the most convenient and effective coating strategy, resulting in a bacterial inhibition of about 53% in respect to the uncoated Ti-6Al-4V disks.
Conclusions: The coating with glutaraldehyde-bound sericin after silanization in the vapor phase showed promising bacterial inhibition values with a significant reduction of S. aureus biofilm. Further studies including higher number of replicates and more peri-implant-relevant microorganisms are needed to evaluate the applicability of this experimental protocol to dental implants.
Keywords: Ti-6Al-4V; bioconjugation; biofilm; coating; dental implants; titanium (Ti).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

