Linking Cancer Metabolic Dysfunction and Genetic Instability through the Lens of Iron Metabolism
- PMID: 31366108
- PMCID: PMC6721799
- DOI: 10.3390/cancers11081077
Linking Cancer Metabolic Dysfunction and Genetic Instability through the Lens of Iron Metabolism
Abstract
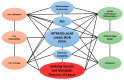
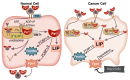
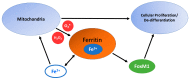
Iron (Fe) is an essential element that plays a fundamental role in a wide range of cellular functions, including cellular proliferation, DNA synthesis, as well as DNA damage and repair. Because of these connections, iron has been strongly implicated in cancer development. Cancer cells frequently have changes in the expression of iron regulatory proteins. For example, cancer cells frequently upregulate transferrin (increasing uptake of iron) and down regulate ferroportin (decreasing efflux of intracellular iron). These changes increase the steady-state level of intracellular redox active iron, known as the labile iron pool (LIP). The LIP typically contains approximately 2% intracellular iron, which primarily exists as ferrous iron (Fe2+). The LIP can readily contribute to oxidative distress within the cell through Fe2+-dioxygen and Fenton chemistries, generating the highly reactive hydroxyl radical (HO•). Due to the reactive nature of the LIP, it can contribute to increased DNA damage. Mitochondrial dysfunction in cancer cells results in increased steady-state levels of hydrogen peroxide and superoxide along with other downstream reactive oxygen species. The increased presence of H2O2 and O2•- can increase the LIP, contributing to increased mitochondrial uptake of iron as well as genetic instability. Thus, iron metabolism and labile iron pools may play a central role connecting the genetic mutational theories of cancer to the metabolic theories of cancer.
Keywords: cancer; ferritin; genetic instability; genetic theory of cancer; iron metabolism; labile iron pool; metabolism; mitochondria; mitochondrial iron; transferrin receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

