Protective Effects of Dendropanax morbifera against Cisplatin-Induced Nephrotoxicity without Altering Chemotherapeutic Efficacy
- PMID: 31366146
- PMCID: PMC6721194
- DOI: 10.3390/antiox8080256
Protective Effects of Dendropanax morbifera against Cisplatin-Induced Nephrotoxicity without Altering Chemotherapeutic Efficacy
Abstract
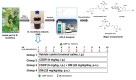
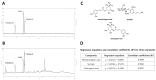
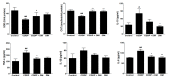
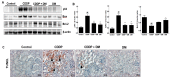
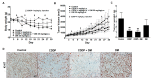
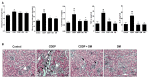
Use of the chemotherapeutic agent cisplatin (CDDP) in cancer patients is limited by the occurrence of acute kidney injury (AKI); however, no protective therapy is available. We aimed to investigate the renoprotective effects of Dendropanax morbifera water extract (DM) on CDDP-induced AKI. Male Sprague-Dawley rats (six animals/group) received: Vehicle (control); CDDP (6 mg/kg, intraperitoneally (i.p.); DM (25 mg/kg, oral); or DM + CDDP injection. CDDP treatment significantly increased blood urea nitrogen (BUN), serum creatinine (sCr), and pro-inflammatory cytokines (IL-6 and TNF-α), and severely damaged the kidney architecture. Urinary excretion of protein-based AKI biomarkers also increased in the CDDP-treated group. In contrast, DM ameliorated CDDP-induced AKI biomarkers. It markedly protected against CDDP-induced oxidative stress by increasing the activity of endogenous antioxidants and reducing the levels of pro-inflammatory cytokines (IL-6 and TNF-α). The protective effect of DM in the proximal tubules was evident upon histopathological examination. In a tumor xenograft model, administration of DM enhanced the chemotherapeutic activity of CDDP and exhibited renoprotective effects against CDDP-induced nephrotoxicity without altering chemotherapeutic efficacy. Our data demonstrate that DM may be an adjuvant therapy with CDDP in solid tumor patients to preserve renal function.
Keywords: Dendropanax morbifera; antioxidants; chemotherapy; cisplatin; renoprotective effect; xenograft model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
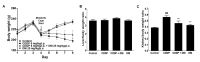



References
-
- Meyer K.B., Madias N.E. Cisplatin nephrotoxicity. Miner. Electrolyte Metab. 1994;20:201–213. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

