Proteomic and histopathological characterisation of sicca subjects and primary Sjögren's syndrome patients reveals promising tear, saliva and extracellular vesicle disease biomarkers
- PMID: 31366407
- PMCID: PMC6670195
- DOI: 10.1186/s13075-019-1961-4
Proteomic and histopathological characterisation of sicca subjects and primary Sjögren's syndrome patients reveals promising tear, saliva and extracellular vesicle disease biomarkers
Abstract
Background: Mononuclear cell infiltration of exocrine glands, production of Ro/SSA and La/SSB autoantibodies, along with oral and ocular dryness, are characteristic features of primary Sjögren's syndrome (pSS). Non-SS sicca subjects, an underexplored group in relation to pSS, display similar sicca symptoms, with possible mild signs of inflammation in their salivary glands, yet with no serological detection of autoantibody production. In this study, we investigated inflammatory manifestations in the salivary gland tissue, tear fluid and saliva of non-SS subjects, as compared to pSS patients and healthy individuals.
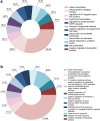
Methods: Fifteen non-SS, 10 pSS and 10 healthy subjects were included in the analyses. Histological evaluation of salivary gland biopsies was performed. Liquid chromatography-mass spectrometry (LC-MS) was conducted on tear fluid and stimulated whole saliva, and proteomic biomarker profiles were generated. Extracellular vesicle (EVs) isolation and characterisation from both fluids were also combined with LC-MS. The LC-MS data were analysed for quantitative differences between patient and control groups using Scaffold. Database for Annotation, Visualization and Integrated Discovery (DAVID) and Functional Enrichment Analysis Tool (FunRich) were applied for functional analyses.
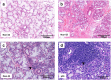
Results: Histopathological evaluation of salivary gland biopsies showed implications of milder inflammation in non-SS subjects through mononuclear cell infiltration, fibrosis and fatty replacement, as compared to pSS patients. Although unaffected in the non-SS group, upregulation of proinflammatory pathways and proteins involved in ubiquitination (LMO7 and HUWE1) and B cell differentiation (TPD52) were detected in tear fluid of pSS patients. Moreover, overexpression of proteins STOM, ANXA4 and ANXA1, regulating cellular innate and adaptive immunological pathways, were further identified in EVs from tear fluid of pSS patients. Finally, whole saliva and EVs isolated from whole saliva of pSS patients expressed proteins vital for innate MHC class I cellular regulation (NGAL) and T cell activation (CD44).
Conclusions: Non-SS sicca subjects may show implications of mild inflammation in their glandular tissue, while their protein profile was strikingly more similar to healthy controls than to pSS patients. Hence, the tear and salivary biomarkers identified could be implemented as potential non-invasive diagnostic tools that may aid in increasing diagnostic accuracy when evaluating non-SS subjects and pSS patients and monitoring disease progression.
Keywords: Adaptive immunity; Autoimmunity; Biomarkers; Extracellular vesicles; Innate immunity; Proteomics; Saliva; Sicca subjects; Sjögren’s syndrome; Tears.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. - DOI - PMC - PubMed
-
- Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, Rasmussen A, Scofield H, Vitali C, Bowman SJ, et al. American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. 2016;2017(76):9–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

