8-Aminoquinoline Therapy for Latent Malaria
- PMID: 31366609
- PMCID: PMC6750137
- DOI: 10.1128/CMR.00011-19
8-Aminoquinoline Therapy for Latent Malaria
Abstract
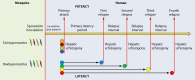
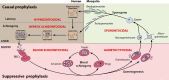
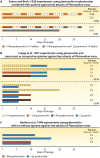
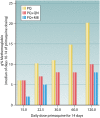
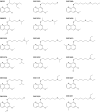
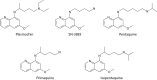
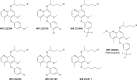
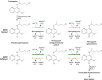
The technical genesis and practice of 8-aminoquinoline therapy of latent malaria offer singular scientific, clinical, and public health insights. The 8-aminoquinolines brought revolutionary scientific discoveries, dogmatic practices, benign neglect, and, finally, enduring promise against endemic malaria. The clinical use of plasmochin-the first rationally synthesized blood schizontocide and the first gametocytocide, tissue schizontocide, and hypnozoitocide of any kind-commenced in 1926. Plasmochin became known to sometimes provoke fatal hemolytic crises. World War II delivered a newer 8-aminoquinoline, primaquine, and the discovery of glucose-6-phosphate dehydrogenase (G6PD) deficiency as the basis of its hemolytic toxicity came in 1956. Primaquine nonetheless became the sole therapeutic option against latent malaria. After 40 years of fitful development, in 2018 the U.S. Food and Drug Administration registered the 8-aminoquinoline called tafenoquine for the prevention of all malarias and the treatment of those that relapse. Tafenoquine also cannot be used in G6PD-unknown or -deficient patients. The hemolytic toxicity of the 8-aminoquinolines impedes their great potential, but this problem has not been a research priority. This review explores the complex technical dimensions of the history of 8-aminoquinolines. The therapeutic principles thus examined may be leveraged in improved practice and in understanding the bright prospect of discovery of newer drugs that cannot harm G6PD-deficient patients.
Keywords: 8-aminoquinolines; CYP2D6; G6PD deficiency; Plasmodium vivax; hemolytic toxicity; latency; plasmochin; primaquine; tafenoquine; therapy.
Copyright © 2019 American Society for Microbiology.
Figures








References
-
- Battle KE, Lucas TCD, Nguyen M, Howes RE, Nandi AK, Twohig KA, Pfeffer DA, Cameron E, Rao PJ, Casey D, Gibson HS, Rozier JA, Dalrymple U, Keddie SH, Collins EL, Harris JR, Guerra CA, Thorn MP, Bisanzio D, Fullman N, Huynh CK, Kulikoff X, Kutz MJ, Lopez AD, Mokdad AH, Naghavi M, Nguyen G, Shackelford KA, Vos T, Wang H, Lim SS, Murray CJL, Price RN, Baird JK, Smith DL, Bhatt S, Weiss DJ, Hay SI, Gething PW. 19 June 2019. Mapping the global endemicity and clinical burden of Plasmodium vivax, 2000-17: a spatial and temporal modelling study. Lancet doi: 10.1016/S0140-6736(19)31096-7. - DOI - PMC - PubMed
-
- World Health Organization. 2015. Control and elimination of Plasmodium vivax malaria: a technical brief, p 64 World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

