Functional analyses of ancestral thioredoxins provide insights into their evolutionary history
- PMID: 31366732
- PMCID: PMC6755812
- DOI: 10.1074/jbc.RA119.009718
Functional analyses of ancestral thioredoxins provide insights into their evolutionary history
Abstract
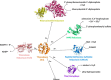
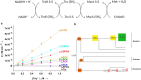
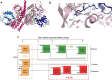
Thioredoxin (Trx) is a conserved, cytosolic reductase in all known organisms. The enzyme receives two electrons from NADPH via thioredoxin reductase (TrxR) and passes them on to multiple cellular reductases via disulfide exchange. Despite the ubiquity of thioredoxins in all taxa, little is known about the functions of resurrected ancestral thioredoxins in the context of a modern mesophilic organism. Here, we report on functional in vitro and in vivo analyses of seven resurrected Precambrian thioredoxins, dating back 1-4 billion years, in the Escherichia coli cytoplasm. Using synthetic gene constructs for recombinant expression of the ancestral enzymes, along with thermodynamic and kinetic assays, we show that all ancestral thioredoxins, as today's thioredoxins, exhibit strongly reducing redox potentials, suggesting that thioredoxins served as catalysts of cellular reduction reactions from the beginning of evolution, even before the oxygen catastrophe. A detailed, quantitative characterization of their interactions with the electron donor TrxR from Escherichia coli and the electron acceptor methionine sulfoxide reductase, also from E. coli, strongly hinted that thioredoxins and thioredoxin reductases co-evolved and that the promiscuity of thioredoxins toward downstream electron acceptors was maintained during evolution. In summary, our findings suggest that thioredoxins evolved high specificity for their sole electron donor TrxR while maintaining promiscuity to their multiple electron acceptors.
Keywords: electron transfer; methionine sulfoxide reductase; molecular evolution; oxidative stress; phylogenetic reconstruction; redox biology; redox homeostasis; thioredoxin; thioredoxin reductase.
© 2019 Napolitano et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

