The bicarbonate/carbon dioxide pair increases hydrogen peroxide-mediated hyperoxidation of human peroxiredoxin 1
- PMID: 31366734
- PMCID: PMC6755790
- DOI: 10.1074/jbc.RA119.008825
The bicarbonate/carbon dioxide pair increases hydrogen peroxide-mediated hyperoxidation of human peroxiredoxin 1
Abstract
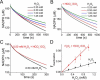
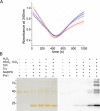
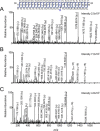
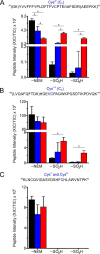
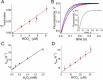
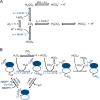
2-Cys peroxiredoxins (Prxs) rapidly reduce H2O2, thereby acting as antioxidants and also as sensors and transmitters of H2O2 signals in cells. Interestingly, eukaryotic 2-Cys Prxs lose their peroxidase activity at high H2O2 levels. Under these conditions, H2O2 oxidizes the sulfenic acid derivative of the Prx peroxidatic Cys (CPSOH) to the sulfinate (CPSO2-) and sulfonated (CPSO3-) forms, redirecting the CPSOH intermediate from the catalytic cycle to the hyperoxidation/inactivation pathway. The susceptibility of 2-Cys Prxs to hyperoxidation varies greatly and depends on structural features that affect the lifetime of the CPSOH intermediate. Among the human Prxs, Prx1 has an intermediate susceptibility to H2O2 and was selected here to investigate the effect of a physiological concentration of HCO3-/CO2 (25 mm) on its hyperoxidation. Immunoblotting and kinetic and MS/MS experiments revealed that HCO3-/CO2 increases Prx1 hyperoxidation and inactivation both in the presence of excess H2O2 and during enzymatic (NADPH/thioredoxin reductase/thioredoxin) and chemical (DTT) turnover. We hypothesized that the stimulating effect of HCO3-/CO2 was due to HCO4-, a peroxide present in equilibrated solutions of H2O2 and HCO3-/CO2 Indeed, additional experiments and calculations uncovered that HCO4- oxidizes CPSOH to CPSO2- with a second-order rate constant 2 orders of magnitude higher than that of H2O2 ((1.5 ± 0.1) × 105 and (2.9 ± 0.2) × 103 m-1·s-1, respectively) and that HCO4- is 250 times more efficient than H2O2 at inactivating 1% Prx1 per turnover. The fact that the biologically ubiquitous HCO3-/CO2 pair stimulates Prx1 hyperoxidation and inactivation bears relevance to Prx1 functions beyond its antioxidant activity.
Keywords: antioxidant defense; bicarbonate; carbon dioxide; hydrogen peroxide; hyperoxidation; inactivation; peroxiredoxin; peroxymonocarbonate; redox signaling; thiol; thiol oxidation.
© 2019 Truzzi et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

Similar articles
-
Enhanced hyperoxidation of peroxiredoxin 2 and peroxiredoxin 3 in the presence of bicarbonate/CO2.Free Radic Biol Med. 2019 Dec;145:1-7. doi: 10.1016/j.freeradbiomed.2019.09.010. Epub 2019 Sep 12. Free Radic Biol Med. 2019. PMID: 31521665
-
Hyperoxidation of peroxiredoxins 2 and 3: rate constants for the reactions of the sulfenic acid of the peroxidatic cysteine.J Biol Chem. 2013 May 17;288(20):14170-14177. doi: 10.1074/jbc.M113.460881. Epub 2013 Mar 29. J Biol Chem. 2013. PMID: 23543738 Free PMC article.
-
Novel hyperoxidation resistance motifs in 2-Cys peroxiredoxins.J Biol Chem. 2018 Jul 27;293(30):11901-11912. doi: 10.1074/jbc.RA117.001690. Epub 2018 Jun 8. J Biol Chem. 2018. PMID: 29884768 Free PMC article.
-
Hyperoxidation of Peroxiredoxins: Gain or Loss of Function?Antioxid Redox Signal. 2018 Mar 1;28(7):574-590. doi: 10.1089/ars.2017.7214. Epub 2017 Sep 8. Antioxid Redox Signal. 2018. PMID: 28762774 Review.
-
Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger H₂O₂, and protein chaperones.Antioxid Redox Signal. 2011 Aug 1;15(3):781-94. doi: 10.1089/ars.2010.3393. Epub 2011 Mar 31. Antioxid Redox Signal. 2011. PMID: 20919930 Review.
Cited by
-
AMP-Activated Protein Kinase (AMPK) at the Crossroads Between CO2 Retention and Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease (COPD).Int J Mol Sci. 2020 Jan 31;21(3):955. doi: 10.3390/ijms21030955. Int J Mol Sci. 2020. PMID: 32023946 Free PMC article. Review.
-
The Peroxidatic Thiol of Peroxiredoxin 1 is Nitrosated by Nitrosoglutathione but Coordinates to the Dinitrosyl Iron Complex of Glutathione.Antioxidants (Basel). 2020 Mar 25;9(4):276. doi: 10.3390/antiox9040276. Antioxidants (Basel). 2020. PMID: 32218363 Free PMC article.
-
Hydrogen peroxide signaling via its transformation to a stereospecific alkyl hydroperoxide that escapes reductive inactivation.Nat Commun. 2021 Nov 16;12(1):6626. doi: 10.1038/s41467-021-26991-5. Nat Commun. 2021. PMID: 34785665 Free PMC article.
-
Carbon dioxide redox metabolites in oxidative eustress and oxidative distress.Biophys Rev. 2021 Nov 6;13(6):889-891. doi: 10.1007/s12551-021-00860-3. eCollection 2021 Dec. Biophys Rev. 2021. PMID: 35059013 Free PMC article.
-
Reactive oxygen species (ROS) as pleiotropic physiological signalling agents.Nat Rev Mol Cell Biol. 2020 Jul;21(7):363-383. doi: 10.1038/s41580-020-0230-3. Epub 2020 Mar 30. Nat Rev Mol Cell Biol. 2020. PMID: 32231263 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

