Lysine harvesting is an antioxidant strategy and triggers underground polyamine metabolism
- PMID: 31367038
- PMCID: PMC6774798
- DOI: 10.1038/s41586-019-1442-6
Lysine harvesting is an antioxidant strategy and triggers underground polyamine metabolism
Abstract
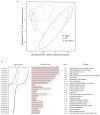
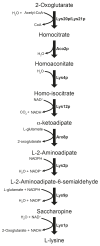
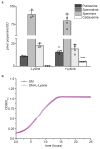
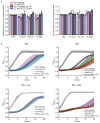
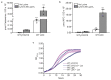
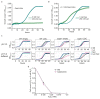
Both single and multicellular organisms depend on anti-stress mechanisms that enable them to deal with sudden changes in the environment, including exposure to heat and oxidants. Central to the stress response are dynamic changes in metabolism, such as the transition from the glycolysis to the pentose phosphate pathway-a conserved first-line response to oxidative insults1,2. Here we report a second metabolic adaptation that protects microbial cells in stress situations. The role of the yeast polyamine transporter Tpo1p3-5 in maintaining oxidant resistance is unknown6. However, a proteomic time-course experiment suggests a link to lysine metabolism. We reveal a connection between polyamine and lysine metabolism during stress situations, in the form of a promiscuous enzymatic reaction in which the first enzyme of the polyamine pathway, Spe1p, decarboxylates lysine and forms an alternative polyamine, cadaverine. The reaction proceeds in the presence of extracellular lysine, which is taken up by cells to reach concentrations up to one hundred times higher than those required for growth. Such extensive harvest is not observed for the other amino acids, is dependent on the polyamine pathway and triggers a reprogramming of redox metabolism. As a result, NADPH-which would otherwise be required for lysine biosynthesis-is channelled into glutathione metabolism, leading to a large increase in glutathione concentrations, lower levels of reactive oxygen species and increased oxidant tolerance. Our results show that nutrient uptake occurs not only to enable cell growth, but when the nutrient availability is favourable it also enables cells to reconfigure their metabolism to preventatively mount stress protection.
Figures




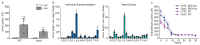

Comment in
-
Yeast cells handle stress by reprogramming their metabolism.Nature. 2019 Aug;572(7768):184-185. doi: 10.1038/d41586-019-02288-y. Nature. 2019. PMID: 31384051 No abstract available.
References
-
- Kuehne A, et al. Acute Activation of Oxidative Pentose Phosphate Pathway as First-Line Response to Oxidative Stress in Human Skin Cells. Mol Cell. 2015;59:359–371. - PubMed
-
- Mima S, et al. Identification of the TPO1 gene in yeast, and its human orthologue TETRAN, which cause resistance to NSAIDs. FEBS Lett. 2007;581:1457–1463. - PubMed
-
- Albertsen M, Bellahn I, Kramer R, Waffenschmidt S. Localization and function of the yeast multidrug transporter Tpo1p. J Biol Chem. 2003;278:12820–12825. - PubMed
-
- Uemura T, Tachihara K, Tomitori H, Kashiwagi K, Igarashi K. Characteristics of the polyamine transporter TPO1 and regulation of its activity and cellular localization by phosphorylation. J Biol Chem. 2005;280:9646–9652. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

