Dietary methionine influences therapy in mouse cancer models and alters human metabolism
- PMID: 31367041
- PMCID: PMC6951023
- DOI: 10.1038/s41586-019-1437-3
Dietary methionine influences therapy in mouse cancer models and alters human metabolism
Abstract
Nutrition exerts considerable effects on health, and dietary interventions are commonly used to treat diseases of metabolic aetiology. Although cancer has a substantial metabolic component1, the principles that define whether nutrition may be used to influence outcomes of cancer are unclear2. Nevertheless, it is established that targeting metabolic pathways with pharmacological agents or radiation can sometimes lead to controlled therapeutic outcomes. By contrast, whether specific dietary interventions can influence the metabolic pathways that are targeted in standard cancer therapies is not known. Here we show that dietary restriction of the essential amino acid methionine-the reduction of which has anti-ageing and anti-obesogenic properties-influences cancer outcome, through controlled and reproducible changes to one-carbon metabolism. This pathway metabolizes methionine and is the target of a variety of cancer interventions that involve chemotherapy and radiation. Methionine restriction produced therapeutic responses in two patient-derived xenograft models of chemotherapy-resistant RAS-driven colorectal cancer, and in a mouse model of autochthonous soft-tissue sarcoma driven by a G12D mutation in KRAS and knockout of p53 (KrasG12D/+;Trp53-/-) that is resistant to radiation. Metabolomics revealed that the therapeutic mechanisms operate via tumour-cell-autonomous effects on flux through one-carbon metabolism that affects redox and nucleotide metabolism-and thus interact with the antimetabolite or radiation intervention. In a controlled and tolerated feeding study in humans, methionine restriction resulted in effects on systemic metabolism that were similar to those obtained in mice. These findings provide evidence that a targeted dietary manipulation can specifically affect tumour-cell metabolism to mediate broad aspects of cancer outcome.
Conflict of interest statement
CONFLICT OF INTEREST
J.W.L and X.G. have patents related to targeting amino acid metabolism in cancer therapy. D.G.K. is a co-founder and has equity in XRAD therapeutics, a company developing radiosensitizing agents. He also has patents related to radiosensitizing agents.
Figures


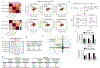
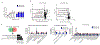




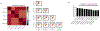
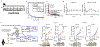

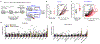
Comment in
-
Commentary: Dietary methionine influences therapy in mouse cancer models and alters human metabolism.Front Oncol. 2020 Jul 6;10:1071. doi: 10.3389/fonc.2020.01071. eCollection 2020. Front Oncol. 2020. PMID: 32733800 Free PMC article. No abstract available.
References
-
- Goncalves MD, Hopkins BD & Cantley LC Dietary Fat and Sugar in Promoting Cancer Development and Progression. Annual Review of Cancer Biology 3, 255–273, doi: 10.1146/annurev-cancerbio-030518-055855 (2019). - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

