Metastatic cancer cell attachment to endothelium is promoted by endothelial glycocalyx sialic acid degradation
- PMID: 31367063
- PMCID: PMC6668365
- DOI: 10.1002/aic.16634
Metastatic cancer cell attachment to endothelium is promoted by endothelial glycocalyx sialic acid degradation
Abstract
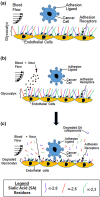
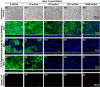
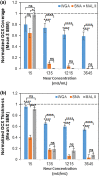
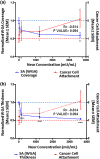
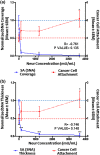
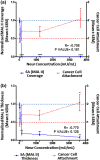
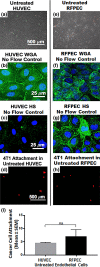
While it is known that cancer cell interactions with vascular endothelial cells (ECs) drive metastatic cancer cell extravasation from blood vessels into secondary tumor sites, the mechanisms of action are still poorly understood. Here, we tested the hypothesis that neuraminidase-induced degradation of EC surface glycocalyx (GCX), particularly the sialic acid (SA) residue components of the GCX, will substantially increase metastatic cancer cell attachment to ECs. To our knowledge, our study is the first to isolate the role of GCX SA residues in cancer cell attachment to the endothelium, which were found to be differentially affected by the presence of neuraminidase and to indeed regulate metastatic cancer cell homing to ECs. We hope that this work will eventually translate to identification of EC GCX-based cancer markers that can be therapeutically targeted to hinder the progression of metastasis.
Keywords: endothelial glycocalyx; metastatic cancer cells; secondary tumor; sialic acid.
Figures
References
-
- Tian Q, Wang Y, Guo H, et al. Recent perspectives of management of breast cancer metastasis ‐ an update. J BUON. 2017;22(2):295‐300. - PubMed
-
- Sharma R, Sharma R, Khaket TP, Dutta C, Chakraborty B, Mukherjee TK. Breast cancer metastasis: putative therapeutic role of vascular cell adhesion molecule‐1. Cell Oncol (Dordr). 2017;40(3):199‐208. - PubMed
-
- Reymond N, d'Agua BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer. 2013;13(12):858‐870. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
