A synthetic chemist's guide to electroanalytical tools for studying reaction mechanisms
- PMID: 31367303
- PMCID: PMC6615219
- DOI: 10.1039/c9sc01545k
A synthetic chemist's guide to electroanalytical tools for studying reaction mechanisms
Abstract
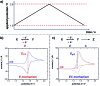
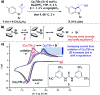
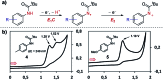
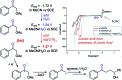
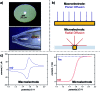
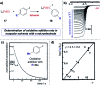
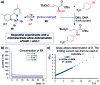
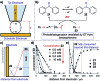
Monitoring reactive intermediates can provide vital information in the study of synthetic reaction mechanisms, enabling the design of new catalysts and methods. Many synthetic transformations are centred on the alteration of oxidation states, but these redox processes frequently pass through intermediates with short life-times, making their study challenging. A variety of electroanalytical tools can be utilised to investigate these redox-active intermediates: from voltammetry to in situ spectroelectrochemistry and scanning electrochemical microscopy. This perspective provides an overview of these tools, with examples of both electrochemically-initiated processes and monitoring redox-active intermediates formed chemically in solution. The article is designed to introduce synthetic organic and organometallic chemists to electroanalytical techniques and their use in probing key mechanistic questions.
Figures
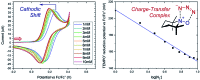
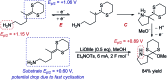
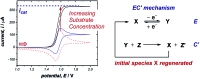


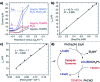
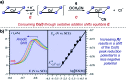
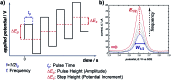
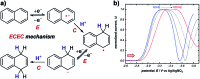
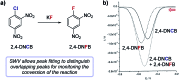
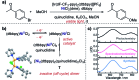
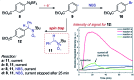
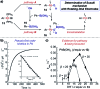
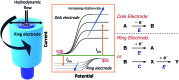


References
-
- Shaw M. H., Twilton J., MacMillan D. W. C. J. Org. Chem. 2016;81:6898–6926. - PMC - PubMed
- Skubi K. L., Blum T. R., Yoon T. P. Chem. Rev. 2016;116:10035–10074. - PMC - PubMed
- Romero N. A., Nicewicz D. A. Chem. Rev. 2016;116:10075–10166. - PubMed
- Staveness D., Bosque I., Stephenson C. R. J. Acc. Chem. Res. 2016;49:2295–2306. - PMC - PubMed
-
- Bard A. J. and Faulkner L. R., Electrochemical Methods, John Wiley & Sons, Inc., Hoboken NJ, 2nd edn, 2001.
- Organic Electrochemistry, ed. O. Hammerich and B. Speiser, Taylor & Francis Group, Boca Raton FL, 5th edn, 2016.
- Jutand A. Chem. Rev. 2008;108:2300–2347. - PubMed
- Savéant J.-M. Chem. Rev. 2008;108:2348–2378. - PubMed
- Costentin C., Drouet S., Passard G., Robert M., Savéant J.-M. J. Am. Chem. Soc. 2013;135:9023–9031. - PubMed
- Rountree E. S., McCarthy B. D., Eisenhart T. T., Dempsey J. L. Inorg. Chem. 2014;53:9983–10002. - PubMed
- Rountree E. S., Martin D. J., McCarthy B. D., Dempsey J. L. ACS Catal. 2016;6:3326–3335.
- Elgrishi N., McCarthy B. D., Rountree E. S., Dempsey J. L. ACS Catal. 2016;6:3644–3659.
- Passard G., Ullman A. M., Brodsky C. N., Nocera D. G. J. Am. Chem. Soc. 2016;138:2925–2928. - PubMed
- Costentin C., Robert M., Savéant J.-M. Curr. Opin. Electrochem. 2017;2:26–31.
-
- Savéant J.-M., Elements of Molecular and Biomolecular Electrochemistry, John Wiley & Sons, Inc., Hoboken NJ, 2006.
- Elgrishi N., Rountree K. J., McCarthy B. D., Rountree E. S., Eisenhart T. T., Dempsey J. L. J. Chem. Educ. 2018;95:197–206.
- Graham D. J., Standard Operating Procedures for Cyclic Voltammetry, 2nd edn, 2018.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

