The effect of a rapid molecular blood test on the use of antibiotics for nosocomial sepsis: a randomized clinical trial
- PMID: 31367384
- PMCID: PMC6647273
- DOI: 10.1186/s40560-019-0391-3
The effect of a rapid molecular blood test on the use of antibiotics for nosocomial sepsis: a randomized clinical trial
Abstract
Background: Appropriate use of antimicrobials is essential to improve outcomes in sepsis. The aim of this study was to determine whether the use of a rapid molecular blood test-SeptiFast (SF) reduces the antibiotic consumption through early de-escalation in patients with nosocomial sepsis compared with conventional blood cultures (BCs).
Methods: This was a prospective, randomized, superiority, controlled trial conducted at Sao Paulo Heart Institute in the period October 2012-May 2016. Adult patients admitted to the hospital for at least 48 h with a diagnosis of nosocomial sepsis underwent microorganism identification by both SF test and BCs. Patients randomized into the intervention group received antibiotic therapy adjustment according to the results of SF. Patients randomized into the control group received standard antibiotic adjustment according to the results of BCs. The primary endpoint was antimicrobial consumption during the first 14 days after randomization.
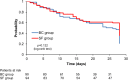
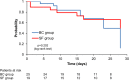
Results: A total of 200 patients were included (100 in each group). The intention to treat analysis found no significant differences in median antibiotic consumption. In the subgroup of patients with positive SF and blood cultures (19 and 25 respectively), we found a statistically significant reduction in the median antimicrobial consumption which was 1429 (1071-2000) days of therapy (DOT)/1000 patients-day in the intervention group and 1889 (1357-2563) DOT/1000 patients-day in the control group (p = 0.017), in the median time of antimicrobial de-escalation (8 versus 54 h-p < 0.001), in the duration of antimicrobial therapy (p = 0.039) and in anti-gram-positive antimicrobial costs (p = 0.002). Microorganism identification was possible in 24.5% of patients (45/184) by SF and 21.2% (39/184) by BC (p = 0.45).
Conclusion: This randomized clinical trial showed that the use of a rapid molecular-based pathogen identification test does not reduce the median antibiotic consumption in nosocomial sepsis. However, in patients with positive microbiological tests, the use of SeptiFast reduced antimicrobial consumption through early de-escalation compared to conventional blood cultures. These results were driven by a reduction in the consumption of antimicrobials used for Gram-positive bacteria.
Trial registration: The trial was registered at ClinicalTrials.gov (NCT01450358) on 12th October 2011.
Keywords: Antibiotic therapy; Blood culture; Critical care; Intensive care; Nosocomial infection; Randomized controlled trial; Rapid molecular test; Sepsis.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures
References
-
- Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief. 2011;62:1–8. - PubMed
-
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving Sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous