A phase I/II study of pemetrexed with sirolimus in advanced, previously treated non-small cell lung cancer
- PMID: 31367538
- PMCID: PMC6626857
- DOI: 10.21037/tlcr.2019.04.19
A phase I/II study of pemetrexed with sirolimus in advanced, previously treated non-small cell lung cancer
Abstract
Background: Single-agent pemetrexed is a treatment for recurrent non-squamous non-small cell lung cancer (NSCLC) that provides limited benefit. Preclinical studies showed promising synergistic effects when the mammalian target of rapamycin (mTOR) inhibitor sirolimus was added to pemetrexed.
Methods: This was a single-institution phase I/II study of pemetrexed in combination with sirolimus. The primary endpoint for the phase I was to determine the maximum tolerated dose (MTD) and safety of the combination. The primary endpoint for the phase II portion was to determine the overall response rate at the MTD. Key eligibility criteria included recurrent, metastatic NSCLC, ECOG performance status of 0-2, and adequate organ function. Sirolimus was administered orally daily after an initial loading dose, and pemetrexed was given intravenously on day 1 of every 21-day cycle.
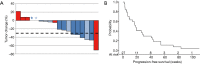
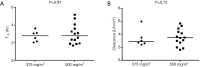
Results: Forty-two patients with recurrent, metastatic NSCLC were enrolled, 22 in phase I and 20 in phase II. The MTD was pemetrexed 500 mg/m2 every 3 weeks, and sirolimus 10 mg on day 1, and 3 mg daily thereafter. Treatment-related adverse events (AEs) occurred in 38 (90.5%) patients. The most common grade 3-4 treatment-related AEs were lymphopenia (31%) and hypophosphatemia (19%). Two treatment-related deaths occurred due to febrile neutropenia and infection, respectively. Among 27 total patients treated at the MTD, 6 (22.2%) had a partial response (PR), 12 (44.4%) had stable disease (SD) and 5 (18.5%) had progressive disease. Median progression-free survival (PFS) was 18.4 weeks (95% CI: 7.0-29.4).
Conclusions: The combination of pemetrexed and sirolimus is active in heavily-pretreated NSCLC (ClinicalTrials.gov Identifier: NCT00923273).
Keywords: Lung cancer; pemetrexed; phase I/II, sirolimus; thymidylate synthase (TS).
Conflict of interest statement
Conflicts of Interest: Phillip A. Dennis is employed by Astrazeneca and owns its stocks. Marc S. Ballas is employed by GlaxoSmithKline and receives personal fees from Astrazeneca and Bristol Myers Squibb. The other authors have no conflicts of interest to declare.
Figures





References
-
- Surveillance Epidemiology and End Results. Available online: www.seer.cancer.gov. Accessed November 16, 2018.
-
- World Health Organization: Cancer, Key Facts. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/. Accessed November 16, 2018.
-
- Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 2000;18:2354-62. 10.1200/JCO.2000.18.12.2354 - DOI - PubMed
-
- Keedy VL, Temin S, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) Mutation testing for patients with advanced non-small- cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol 2011;29:2121-7. 10.1200/JCO.2010.31.8923 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
