Omadacycline for Acute Bacterial Skin and Skin Structure Infections
- PMID: 31367742
- PMCID: PMC6669297
- DOI: 10.1093/cid/ciz396
Omadacycline for Acute Bacterial Skin and Skin Structure Infections
Abstract
Background: Within the last decade, methicillin-resistant Staphylococcus aureus (MRSA) has emerged as a frequent cause of purulent skin and soft tissue infections. New therapeutic options are being investigated for these infections.
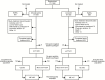
Methods: We report an integrated analysis of 2 randomized, controlled studies involving omadacycline, a novel aminomethylcycline, and linezolid for the treatment of acute bacterial skin and skin structure infections (ABSSSI). Omadacycline in Acute Skin and Skin Structure Infections Study 1 (OASIS-1) initiated patients on intravenous omadacycline or linezolid, with the option to transition to an oral formulation after day 3. OASIS-2 was an oral-only study of omadacycline versus linezolid.
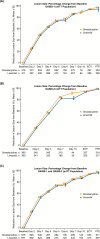
Results: In total, 691 patients received omadacycline and 689 patients received linezolid. Infection types included wound infection in 46.8% of patients, cellulitis/erysipelas in 30.5%, and major abscess in 22.7%. Pathogens were identified in 73.2% of patients. S. aureus was detected in 74.7% and MRSA in 32.4% of patients in whom a pathogen was identified. Omadacycline was noninferior to linezolid using the Food and Drug Administration primary endpoint of early clinical response (86.2% vs 83.9%; difference 2.3, 95% confidence interval -1.5 to 6.2) and using the European Medicines Agency primary endpoint of investigator-assessed clinical response at the posttreatment evaluation. Clinical responses were similar across different infection types and infections caused by different pathogens. Treatment-emergent adverse events, mostly described as mild or moderate, were reported by 51.1% of patients receiving omadacycline and 41.2% of those receiving linezolid.
Conclusions: Omadacycline was effective and safe in ABSSSI.
Clinical trials registration: NCT02378480 and NCT02877927.
Keywords: MRSA; acute bacterial skin and skin structure infections; omadacycline; skin infection; tetracyclines.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

References
-
- Yanai H, Hamasaki H, Tsuda N, et al. . Group B streptococcus infection and diabetes: a review. J Microbiol Antimicrob 2012; 4:1–5.

