Introducing FDG PET/CT-guided chemoradiotherapy for stage III NSCLC in low- and middle-income countries: preliminary results from the IAEA PERTAIN trial
- PMID: 31367906
- PMCID: PMC6717604
- DOI: 10.1007/s00259-019-04421-5
Introducing FDG PET/CT-guided chemoradiotherapy for stage III NSCLC in low- and middle-income countries: preliminary results from the IAEA PERTAIN trial
Abstract
Purpose: Patients with stage III non-small-cell lung cancer (NSCLC) treated with chemoradiotherapy (CRT) in low- and middle-income countries (LMIC) continue to have a poor prognosis. It is known that FDG PET/CT improves staging, treatment selection and target volume delineation (TVD), and although its use has grown rapidly, it is still not widely available in LMIC. CRT is often used as sequential treatment, but is known to be more effective when given concurrently. The aim of the PERTAIN study was to assess the impact of introducing FDG PET/CT-guided concurrent CRT, supported by training and quality control (QC), on the overall survival (OS) and progression-free survival (PFS) of patients with stage III NSCLC.
Methods: The study included patients with stage III NSCLC from nine medical centres in seven countries. A retrospective cohort was managed according to local practices between January 2010 and July 2014, which involved only optional diagnostic FDG PET/CT for staging (not for TVD), followed by sequential or concurrent CRT. A prospective cohort between August 2015 and October 2018 was treated according to the study protocol including FDG PET/CT in treatment position for staging and multimodal TVD followed by concurrent CRT by specialists trained in protocol-specific TVD and with TVD QC. Kaplan-Meier analysis was used to assess OS and PFS in the retrospective and prospective cohorts.
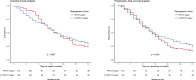
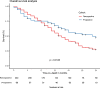
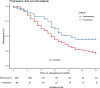
Results: Guidelines for FDG PET/CT image acquisition and TVD were developed and published. All specialists involved in the PERTAIN study received training between June 2014 and May 2016. The PET/CT scanners used received EARL accreditation. In November 2018 a planned interim analysis was performed including 230 patients in the retrospective cohort with a median follow-up of 14 months and 128 patients in the prospective cohort, of whom 69 had a follow-up of at least 1 year. Using the Kaplan-Meier method, OS was significantly longer in the prospective cohort than in the retrospective cohort (23 vs. 14 months, p = 0.012). In addition, median PFS was significantly longer in the prospective cohort than in the retrospective cohort (17 vs. 11 months, p = 0.012).
Conclusion: In the PERTAIN study, the preliminary results indicate that introducing FDG PET/CT-guided concurrent CRT for patients with stage III NSCLC in LMIC resulted in a significant improvement in OS and PFS. The final study results based on complete data are expected in 2020.
Keywords: Low- and middle-income countries; Non-small-cell lung cancer; PET/CT-guided chemoradiotherapy.
Conflict of interest statement
None.
Figures
References
-
- Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. - DOI - PMC - PubMed
-
- Ung YC, Bezjak A, Coakley N, Evans WK; the Lung Cancer Disease Site Group of Cancer Care Ontario. Positron emission tomography with 18fluorodeoxyglucose in radiation treatment planning for non-small cell lung cancer: a systematic review. J Thorac Oncol. 2011;6:86–97.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

