System for application of controlled forces on dental implants in rat maxillae: Influence of the number of load cycles on bone healing
- PMID: 31368244
- PMCID: PMC7078813
- DOI: 10.1002/jbm.b.34449
System for application of controlled forces on dental implants in rat maxillae: Influence of the number of load cycles on bone healing
Abstract
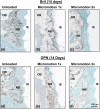
Experimental studies on the effect of micromotion on bone healing around implants are frequently conducted in long bones. In order to more closely reflect the anatomical and clinical environments around dental implants, and eventually be able to experimentally address load-management issues, we have developed a system that allows initial stabilization, protection from external forces, and controlled axial loading of implants. Screw-shaped implants were placed on the edentulous ridge in rat maxillae. Three loading regimens were applied to validate the system; case A no loading (unloaded implant) for 14 days, case B no loading in the first 7 days followed by 7 days of a single, daily loading session (60 cycles of an axial force of 1.5 N/cycle), and case C no loading in the first 7 days followed by 7 days of two such daily loading sessions. Finite element modeling of the peri-implant compressive and tensile strains plus histological and immunohistochemical analyses revealed that in case B any tissue damage resulting from the applied force (and related interfacial strains) did not per se disturb bone healing, however, in case C, the accumulation of damage resulting from the doubling of loading sessions severely disrupted the process. These proof-of-principle results validate the applicability of our system for controlled loading, and provide new evidence on the importance of the number of load cycles applied on healing of maxillary bone.
Keywords: bone; implant; loading; micromotion, maxilla, rat.
© 2019 The Authors. Journal of Biomedical Materials Research Part B: Applied Biomaterials published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no conflicts of interest to report.
Figures






References
-
- Brunski, J. B. (2013). Metals: Basic principles, Chapter 1.2.3, pp. 111–119, in Biomaterials Science: An Introduction to Materials in Medicine, Third Edition. In ASH B. D. R., Schoen F. J., & Lemons J. E. (Eds.), Amsterdam, Netherlands: Elsevier Inc.
-
- Burstone, C. J. , & Goldberg, A. J. (1980). Beta titanium: A new orthodontic alloy. American Journal of Orthodontics, 77(2), 121–132. - PubMed
-
- Carter, D. R. , Beaupré, G. S. (2001). Skeletal Function and Form: Mechanobiology of Skeletal Development, Aging, and Regeneration. Cambridge, United Kingdom: Cambridge University Press, pp. 318.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

