Genomic profile of urine has high diagnostic sensitivity compared to cytology in non-invasive urothelial bladder cancer
- PMID: 31368627
- PMCID: PMC6778642
- DOI: 10.1111/cas.14155
Genomic profile of urine has high diagnostic sensitivity compared to cytology in non-invasive urothelial bladder cancer
Abstract
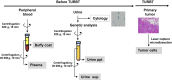
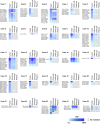
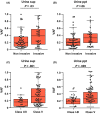
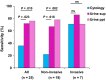
Cytology is widely conducted for diagnosis of urothelial bladder cancer; however, its sensitivity is still low. Recent studies show that liquid biopsies can reflect tumor genomic profiles. We aim to investigate whether plasma or urine is more suitable for detecting tumor-derived DNA in patients with early-stage urothelial bladder cancer. Targeted sequencing of 71 genes was carried out using a total of 150 samples including primary tumor, urine supernatant, urine precipitation, plasma and buffy coat from 25 patients with bladder cancer and five patients with cystitis and benign tumor. We compared mutation profiles between each sample, identified tumor-identical mutations and compared tumor diagnostic sensitivities between urine and conventional cytology. We identified a total of 168 somatic mutations in primary tumor. In liquid biopsies, tumor-identical mutations were found at 53% (89/168) in urine supernatant, 48% (81/168) in urine precipitation and 2% (3/168) in plasma. The high variant allele fraction of urine was significantly related to worse clinical indicators such as tumor invasion and cytological examination. Although conventional cytology detected tumor cells in only 22% of non-invasive tumor, tumor diagnostic sensitivity increased to 67% and 78% using urine supernatant and precipitation, respectively. Urine is an ideal liquid biopsy for detecting tumor-derived DNA and more precisely reflects tumor mutational profiles than plasma. Genomic analysis of urine is clinically useful for diagnosis of superficial bladder cancer at early stage.
Keywords: NGS; diagnosis; liquid biopsy; urine; urothelial bladder cancer.
© 2019 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures

References
-
- Lynch CF, Cohen MB. Urinary system. Cancer. 1995;75:316‐329. - PubMed
-
- Babjuk M, Bohle A, Burger M, et al. EAU guidelines on non‐muscle‐invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017;71:447‐461. - PubMed
-
- Theriault GP, Tremblay CG, Armstrong BG. Bladder cancer screening among primary aluminum production workers in Quebec. J Occup Med. 1990;32:869‐872. - PubMed
-
- Cappellen D, De Oliveira C, Ricol D, et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999;23:18‐20. - PubMed
-
- Platt FM, Hurst CD, Taylor CF, Gregory WM, Harnden P, Knowles MA. Spectrum of phosphatidylinositol 3‐kinase pathway gene alterations in bladder cancer. Clin Cancer Res. 2009;15:6008‐6017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

