Advances in the treatment of hereditary transthyretin amyloidosis: A review
- PMID: 31368669
- PMCID: PMC6749475
- DOI: 10.1002/brb3.1371
Advances in the treatment of hereditary transthyretin amyloidosis: A review
Abstract
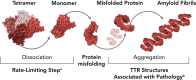
Introduction: Amyloid transthyretin amyloidosis (ATTR) is a progressive and often fatal disease caused by the buildup of mutated (hereditary ATTR [hATTR]; also known as ATTR variant [ATTRv]) or normal transthyretin (wild-type ATTR) throughout the body. Two new therapies-inotersen, an antisense oligonucleotide therapy, and patisiran, an RNA interference therapy-received marketing authorization and represent a significant advance in the treatment of amyloidosis. Herein, we describe the clinical presentation of ATTR, commonly used procedures in its diagnosis, and current treatment landscape for ATTR, with a focus on hATTR.
Methods: A PubMed search from 2008 to September 2018 was conducted to review the literature on ATTR.
Results: Until recently, there have been few treatment options for polyneuropathy of hATTR. Inotersen and patisiran substantially reduce the amyloidogenic precursor protein transthyretin and have demonstrated efficacy in patients with early- and late-stage disease and in slowing or improving neuropathy progression. In contrast, established therapies, such as liver transplantation, typically reserved for patients with early-stage disease, and tafamidis, indicated for the treatment of early-stage disease in Europe, or diflunisal, a nonsteroidal anti-inflammatory drug that is used off-label, are associated with side effects and/or unclear efficacy in certain patient populations. Thus, inotersen and patisiran are positioned to be the preferred therapeutic modalities.
Conclusions: Important differences between inotersen and patisiran, including formulation, dosing, requirements for premedications, and safety monitoring, require an understanding and knowledge of each treatment for informed decision making.
Keywords: amyloid; hATTR; inotersen; patisiran; transthyretin amyloidosis.
© 2019 The Authors. Brain and Behavior published by Wiley Periodicals, Inc.
Figures
References
-
- Adams, D. , Gonzalez‐Duarte, A. , O'Riordan, W. O. , Yang, C. C. , Yamashita, T. , Kristen, A. , … Suhr, O. B. (2018). Evaluation of quality of life and disability in patients with hereditary transthyretin‐mediated (hATTR) amyliodosis with polyneuropathy following treatment with patisrian, an investigational RNAi therapeutic: Results from a phase 3 APOLLO study. Presented at: 70th American Academy of Neurology Annual Meeting; April 21–25, 2018; Los Angeles, CA.
-
- Akcea Therapeutics, Inc. (2018a). Tegsedi injection [prescribing information]. Boston, MA: Akcea Therapeutics, Inc.
-
- Akcea Therapeutics, Inc. (2018b). Product monograph including patient medication information: Tegsedi™ inotersen injection. Retrieved from http://www.akceatx.ca/wp-content/uploads/2018/10/FINAL-Pristine-PM_02OCT...
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

