Delta-like 3 localizes to neuroendocrine cells and plays a pivotal role in gastrointestinal neuroendocrine malignancy
- PMID: 31369178
- PMCID: PMC6778628
- DOI: 10.1111/cas.14157
Delta-like 3 localizes to neuroendocrine cells and plays a pivotal role in gastrointestinal neuroendocrine malignancy
Abstract
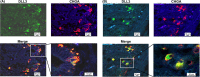
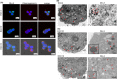
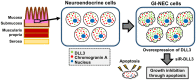
Delta-like 3 (DLL3) is a member of the Delta/Serrate/Lag2 (DSL) group of Notch receptor ligands. Five DSL ligands are known in mammals, among which DLL3 has a unique structure. In the last few years, DLL3 has attracted attention as a novel molecular targeting gene in neuroendocrine carcinoma of the lung due to its high expression. However, the expression pattern and functions of DLL3 in the gastrointestinal tract and gastrointestinal neuroendocrine carcinoma remain unclear. In this study, we examined the expression and role of DLL3 in the gastrointestinal tract, as well as in gastrointestinal neuroendocrine carcinoma. Immunohistochemical staining of the human normal gastrointestinal tract revealed that DLL3 localized in neuroendocrine cells. DLL3 showed intense staining in chromogranin A-positive gastric cancer specimens. Real-time quantitative RT-PCR and western blotting analyses showed considerable upregulation of DLL3 in gastrointestinal neuroendocrine carcinoma cell lines. Immuno-electron microscopy demonstrated abundant expression of DLL3 in neurosecretory granules in these cells. Furthermore, gene silencing of DLL3 caused significant growth inhibition through the induction of intrinsic apoptosis. Our findings suggest that DLL3 is expressed in neuroendocrine cells of the gastrointestinal tract and that it has a pivotal role in gastrointestinal neuroendocrine carcinoma cells. Based on these findings, further investigations are required to achieve a breakthrough in developing therapeutic strategies for gastrointestinal neuroendocrine carcinoma.
Keywords: DLL3; apoptosis; chromogranin A; neuroendocrine; neuroendocrine carcinoma.
© 2019 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures





References
-
- Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer. 2003;3:756‐767. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

