Why flavored vape products may be attractive: Green apple tobacco flavor elicits reward-related behavior, upregulates nAChRs on VTA dopamine neurons, and alters midbrain dopamine and GABA neuron function
- PMID: 31369741
- PMCID: PMC6751572
- DOI: 10.1016/j.neuropharm.2019.107729
Why flavored vape products may be attractive: Green apple tobacco flavor elicits reward-related behavior, upregulates nAChRs on VTA dopamine neurons, and alters midbrain dopamine and GABA neuron function
Abstract
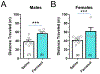
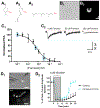
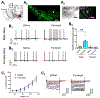
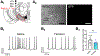
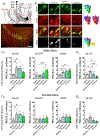
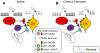
While nicotine is the primary addictive component in tobacco products, additional flavors have become a concern with the growing popularity of electronic nicotine delivery systems (ENDS). For this reason, we have begun to investigate popular tobacco and ENDS flavors. Here, we examined farnesol, a chemical flavorant used in green apple and fruit flavors in ENDS e-liquids, for its ability to produce reward-related behavior. Using male and female 3-6 month old C57BL/6 J mice and farnesol doses of 0.1, 1, and 10 mg/kg we identified a sex-dependent effect in a conditioned place preference assay: farnesol-alone produces reward-related behavior in only male mice. Despite this sex-dependent effect, 1.0 mg/kg farnesol enhances locomotor activity in both male and female mice. To understand farnesol's effect on reward-related behavior, we used whole-cell patch-clamp electrophysiology and confocal microscopy to investigate changes in putative dopamine and GABA neurons. For these approaches, we utilized genetically modified mice that contain fluorescent nicotinic acetylcholine receptors (nAChRs). Our electrophysiological assays with male mice revealed that farnesol treatment increases ventral tegmental area (VTA) dopamine neuron firing frequency and this may be due to a decrease in inhibitory tone from GABA neurons. Our microscopy assays revealed that farnesol treatment produces a significant upregulation of α6* nAChRs in male mice but not female mice. This was supported by an observed increase in α6* nAChR function in additional electrophysiology assays. These data provide evidence that popular tobacco flavorants may alter smoking-related behavior and promote the need to examine additional ENDS flavors.
Keywords: Addiction; Conditioned place preference; Dopamine neuron; Electrophysiology; Flavorants; GABA neuron; Microscopy; Nicotinic receptors; Tobacco; Vaping.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures




References
-
- Antoniou K, Papathanasiou G, Papalexi E, Hyphantis T, Nomikos GG, Spyraki C, Papadopoulou-Daifoti Z (2008) Individual responses to novelty are associated with differences in behavioral and neurochemical profiles. Behav Brain Res 187:462–472. - PubMed
-
- Aszyk J, Kubica P, Wozniak MK, Namiesnik J, Wasik A, Kot-Wasik A (2018) Evaluation of flavour profiles in e-cigarette refill solutions using gas chromatography-tandem mass spectrometry. J Chromatogr A 1547:86–98. - PubMed
-
- Benowitz NL, Samet JM (2011) The threat of menthol cigarettes to U.S. public health. N Engl J Med 364:2179–2181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

