Molecular and Hormonal Aspects of Drought-Triggered Flower Shedding in Yellow Lupine
- PMID: 31370140
- PMCID: PMC6695997
- DOI: 10.3390/ijms20153731
Molecular and Hormonal Aspects of Drought-Triggered Flower Shedding in Yellow Lupine
Abstract
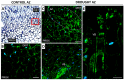
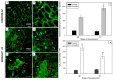
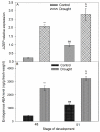
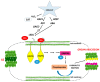
The drought is a crucial environmental factor that determines yielding of many crop species, e.g., Fabaceae, which are a source of valuable proteins for food and feed. Herein, we focused on the events accompanying drought-induced activation of flower abscission zone (AZ)-the structure responsible for flower detachment and, consequently, determining seed production in Lupinus luteus. Therefore, detection of molecular markers regulating this process is an excellent tool in the development of improved drought-resistant cultivars to minimize yield loss. We applied physiological, molecular, biochemical, immunocytochemical, and chromatography methods for a comprehensive examination of changes evoked by drought in the AZ cells. This factor led to significant cellular changes and activated AZ, which consequently increased the flower abortion rate. Simultaneously, drought caused an accumulation of mRNA of genes inflorescence deficient in abscission-like (LlIDL), receptor-like protein kinase HSL (LlHSL), and mitogen-activated protein kinase6 (LlMPK6), encoding succeeding elements of AZ activation pathway. The content of hydrogen peroxide (H2O2), catalase activity, and localization significantly changed which confirmed the appearance of stressful conditions and indicated modifications in the redox balance. Loss of water enhanced transcriptional activity of the abscisic acid (ABA) and ethylene (ET) biosynthesis pathways, which was manifested by elevated expression of zeaxanthin epoxidase (LlZEP), aminocyclopropane-1-carboxylic acid synthase (LlACS), and aminocyclopropane-1-carboxylic acid oxidase (LlACO) genes. Accordingly, both ABA and ET precursors were highly abundant in AZ cells. Our study provides information about several new potential markers of early response on water loss, which can help to elucidate the mechanisms that control plant response to drought, and gives a useful basis for breeders and agronomists to enhance tolerance of crops against the stress.
Keywords: LlHSL; LlIDA; LlMPK6; abscisic acid; abscission zone; catalase; drought stress; ethylene; hormone homeostasis; yellow lupine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- McKeown A.W., Warland J., McDonald M.R. Long-term climate and weather patterns in relation to crop yield: A mini review. Can. J. Bot. 2006;84:1031–1037. doi: 10.1139/b06-080. - DOI
-
- Ainouche A.K., Bayer R.J., Misset M.T. Molecular phylogeny, diversification and character evolution in Lupinus (Fabaceae) with special attention to Mediterranean and African lupines. Plant Syst. Evol. 2004;246:211–222. doi: 10.1007/s00606-004-0149-8. - DOI
-
- Wilmowicz E., Frankowski K., Kućko A., Świdziński M., de Dios Alché J., Nowakowska A., Kopcewicz J. The influence of abscisic acid on the ethylene biosynthesis pathway in the functioning of the flower abscission zone in Lupinus luteus. J. Plant Physiol. 2016;206:49–58. doi: 10.1016/j.jplph.2016.08.018. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

