A Novel Adenosine Kinase from Bombyx mori: Enzymatic Activity, Structure, and Biological Function
- PMID: 31370143
- PMCID: PMC6695918
- DOI: 10.3390/ijms20153732
A Novel Adenosine Kinase from Bombyx mori: Enzymatic Activity, Structure, and Biological Function
Abstract
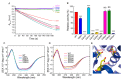
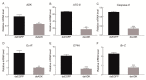
Adenosine kinase (ADK) is the first enzyme in the adenosine remediation pathway that catalyzes adenosine phosphorylation into adenosine monophosphate, thus regulating adenosine homeostasis in cells. To obtain new insights into ADK from Bombyx mori (BmADK), we obtained recombinant BmADK, and analyzed its activity, structure, and function. Gel-filtration showed BmADK was a monomer with molecular weight of approximately 38 kDa. Circular dichroism spectra indicated BmADK had 36.8% α-helix and 29.9% β-strand structures, respectively. The structure of BmADK was stable in pH 5.0-11.0, and not affected under 30 °C. The melting temperature and the enthalpy and entropy changes in the thermal transition of BmADK were 46.51 ± 0.50 °C, 253.43 ± 0.20 KJ/mol, and 0.79 ± 0.01 KJ/(mol·K), respectively. Site-directed mutagenesis demonstrated G68, S201, E229, and D303 were key amino acids for BmADK structure and activity. In particular, S201A mutation significantly increased the α-helix content of BmADK and its activity. BmADK was located in the cytoplasm and highly expressed in the silk gland during the pre-pupal stage. RNA interference revealed the downregulation of BmADK decreased ATG-8, Caspase-9, Ec-R, E74A, and Br-C expression, indicating it was likely involved in 20E signaling, apoptosis, and autophagy to regulate silk gland degeneration and silkworm metamorphosis. Our study greatly expanded the knowledge on the activity, structure, and role of ADK.
Keywords: Bombyx mori; adenosine kinase; enzymatic activity; structure.
Conflict of interest statement
All authors declare there are no conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject of this manuscript.
Figures



References
-
- Singh B., Hao W.H., Wu Z.C., Eigl B., Gupta R.S. Cloning and characterization of cDNA for adenosine kinase from mammalian (Chinese hamster, mouse, human and rat) species: High frequency mutants of Chinese hamster ovary cells involve structural alterations in the gene. Eur. J. Biochem. 1996;241:564–571. doi: 10.1111/j.1432-1033.1996.00564.x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

