A Contactless and Biocompatible Approach for 3D Active Microrobotic Targeted Drug Delivery
- PMID: 31370254
- PMCID: PMC6722705
- DOI: 10.3390/mi10080504
A Contactless and Biocompatible Approach for 3D Active Microrobotic Targeted Drug Delivery
Abstract
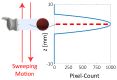
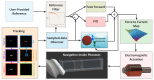
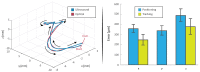
As robotic tools are becoming a fundamental part of present day surgical interventions, microrobotic surgery is steadily approaching clinically-relevant scenarios. In particular, minimally invasive microrobotic targeted drug deliveries are reaching the grasp of the current state-of-the-art technology. However, clinically-relevant issues, such as lack of biocompatibility and dexterity, complicate the clinical application of the results obtained in controlled environments. Consequently, in this work we present a proof-of-concept fully contactless and biocompatible approach for active targeted delivery of a drug-model. In order to achieve full biocompatiblity and contacless actuation, magnetic fields are used for motion control, ultrasound is used for imaging, and induction heating is used for active drug-model release. The presented system is validated in a three-dimensional phantom of human vessels, performing ten trials that mimic targeted drug delivery using a drug-coated microrobot. The system is capable of closed-loop motion control with average velocity and positioning error of 0.3 mm/s and 0.4 mm, respectively. Overall, our findings suggest that the presented approach could augment the current capabilities of microrobotic tools, helping the development of clinically-relevant approaches for active in-vivo targeted drug delivery.
Keywords: magnetic actuation; microrobotics; minimally-invasive surgery; surgical robotics; targeted drug delivery; ultrasound tracking.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Feynman R. Feynman and Computation. CRC Press; Boca Raton, FL, USA: 2018. There’s plenty of room at the bottom; pp. 63–76.
-
- Hosney A., Abdalla J., Amin I.S., Hamdi N., Khalil I.S.M. In vitro validation of clearing clogged vessels using microrobots; Proceedings of the IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob); Singapore. 26–29 June 2016; pp. 272–277.
-
- Zhang Z., Liu J., Wang X., Zhao Q., Zhou C., Tan M., Pu H., Xie S., Sun Y. Robotic Pick-and-Place of Multiple Embryos for Vitrification. IEEE Robot. Autom. Lett. 2017;2:570–576. doi: 10.1109/LRA.2016.2640364. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

