The Neuroprotective Effects of Necrostatin-1 on Subarachnoid Hemorrhage in Rats Are Possibly Mediated by Preventing Blood-Brain Barrier Disruption and RIP3-Mediated Necroptosis
- PMID: 31370690
- PMCID: PMC6802141
- DOI: 10.1177/0963689719867285
The Neuroprotective Effects of Necrostatin-1 on Subarachnoid Hemorrhage in Rats Are Possibly Mediated by Preventing Blood-Brain Barrier Disruption and RIP3-Mediated Necroptosis
Abstract
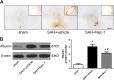
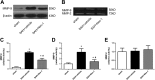
Despite the substantial efforts to elucidate the role of early brain injury in subarachnoid hemorrhage (SAH), an effective pharmaceutical therapy for patients with SAH continues to be unavailable. This study aims to reveal the role of necroptosis after SAH, and explore whether the disruption of the blood-brain barrier (BBB) and RIP3-mediated necroptosis following SAH in a rat SAH model are altered by necrostatin-1 via its selective inhibition of receptor-interacting protein kinase 1 (RIP1). Sixty-five rats were used in the experiments. The SAH model was established using endovascular perforation. Necrostatin-1 was intracerebroventricularly injected 1 h before SAH induction. The neuroprotective effects of necrostatin-1 were evaluated with multiple methods such as magnetic resonance imaging (MRI) scanning, immunohistochemistry, propidium iodide (PI) labeling, and western blotting. Pretreatment with necrostatin-1 attenuated brain swelling and reduced the lesion volume on T2 sequence and ventricular volume on MRI 72 h after SAH induction. Albumin leakage and the degradation of tight junction proteins were also ameliorated by necrostatin-1 administration. In addition, necrostatin-1 decreased the number of PI-positive cells in the basal cortex, reduced the levels of the RIP3 and MLKL proteins, and inhibited the production of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α. Based on the findings from the present study, the selective RIP1 inhibitor necrostatin-1 functioned as a neuroprotective agent after SAH by attenuating brain swelling and BBB disruption. Moreover, the necrostatin-1 pretreatment prevented SAH-induced necroptosis by suppressing the activity of the RIP3/MLKL signaling pathway. These results will provide insights into new drugs and pharmacological targets to manage SAH, which are worth further study.
Keywords: blood–brain barrier; early brain injury; necroptosis; necrostatin-1; subarachnoid hemorrhage.
Conflict of interest statement
Figures






References
-
- Connolly ES, Jr., Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(6):1711–1737. - PubMed
-
- van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain. 2001;124(Pt 2):249–278. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

