Third-line antiretroviral therapy in low-income and middle-income countries (ACTG A5288): a prospective strategy study
- PMID: 31371262
- PMCID: PMC6857629
- DOI: 10.1016/S2352-3018(19)30146-8
Third-line antiretroviral therapy in low-income and middle-income countries (ACTG A5288): a prospective strategy study
Abstract
Background: Antiretroviral therapy (ART) management is challenging for individuals in resource-limited settings presenting for third-line treatment because of complex resistance patterns, partly due to reduced access to viral load monitoring. We aimed to evaluate use of newer antiretroviral drugs and contemporary management approaches, including population-based sequencing, to select appropriate antiretrovirals, plasma viral load monitoring, and interventions to improve adherence in individuals presenting with second-line viral failure.
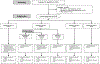
Methods: A5288 was a phase 4, third-line ART strategy study done at 19 urban sites in ten countries that enrolled adult participants with confirmed plasma HIV-1 RNA (viral load) of 1000 copies per mL or more after more than 24 weeks of protease inhibitor-based second-line ART. The primary objective was to use antiretrovirals (raltegravir, etravirine, and ritonavir-boosted darunavir) and diagnostic monitoring technologies, including viral load, genotyping, and adherence support to achieve viral load suppression (defined as ≤200 copies per mL) in 65% or more of participants. ART history and real-time drug resistance genotypes were used to assign participants to one of four cohorts: cohort A (no lopinavir resistance) stayed on second-line ART and cohorts B (B1, best available nucleoside reverse transcriptase inhibitors [NRTIs] plus ritonavir-boosted darunavir plus raltegravir; B2, ritonavir-boosted darunavir plus raltegravir plus etravirine; B3, ritonavir-boosted darunavir, raltegravir, and either tenofovir plus emtricitabine or tenofovir plus lamivudine), C (ritonavir-boosted darunavir plus raltegravir plus tenofovir-emtricitabine or tenofovir plus lamivudine), and D (best available NRTIs plus ritonavir-boosted darunavir plus raltegravir) were defined by increasing levels of resistance and received appropriate regimens, including new antiretrovirals. Participants in Cohort B without detectable hepatitis B surface antigen were assigned by blocked randomisation to cohorts B1 and B2, and those with detectable hepatitis B surface antigen were assigned to cohort B3. The trial is registered with ClinicalTrials.gov, number NCT01641367.
Findings: From Jan 10, 2013, to Sept 10, 2015, 545 participants were enrolled. 287 (53%) were assigned to cohort A, 74 (14%) to B1, 72 (13%) to B2, eight (1%) to B3, 70 (13%) to C, and 34 (6%) to D. Overall, 349 (64%, 95% CI 60-68) participants achieved viral suppression at week 48, with proportions varying from 125 (44%) of 287 in cohort A to 65 (88%) of 74 in cohort B1, 63 (88%) of 72 in B2, eight (100%) of eight in B3, 63 (90%) of 70 in C, and 25 (74%) of 34 in D. Participants in cohort A remained on their second-line protease inhibitor, and had the most participants with grade 3 or higher adverse events (147 [51%]).
Interpretation: Targeted real-time genotyping to select third-line ART can appropriately allocate more costly antiretrovirals to those with greater levels of HIV drug resistance.
Funding: National Institutes of Health.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
ACC reports grants from NIH during the conduct of the study; personal fees from Merck & Co., grants from Bristol-Myers-Squibb, outside the submitted work.
JWM reports grants from NIAID/NIH, during the conduct of the study; personal fees from University of Pittsburgh, grants from Gilead Sciences, grants from Janssen Pharmaceuticals, personal fees from Bristol-Myers Squibb, personal fees from Gilead Sciences, personal fees from Janssen Pharmaceuticals, personal fees from Merck, personal fees from Xi’an Yufan Biotechnologies, other from Cocrystal Pharma, Inc., outside the submitted work; In addition, Dr. Mellors has a patent Patent #: 8,815,829 pending.
CLW reports personal fees from IPM (International Partnership for Microbicides), personal fees from Right-to-Care, personal fees from MSD-MERCK, outside the submitted work.
JR reports grants from NIH during the conduct of the study.
MDH reports grants from NIH during the conduct of the study.
RG reports grants from NIH during the conduct of the study; personal fees from Pfizer, outside the submitted work.
RTS reports grants from National Institute of Allergy and Infectious Diseases, during the conduct of the study; grants from Gilead Sciences, grants and personal fees from Monogram Biosciences, grants from Pfizer, personal fees from CytoDyn, personal fees from VIR, outside the submitted work.
VM reports grants from NIH, during the conduct of the study.
LM reports grants from The National Institute of Allergy and Infectious Diseases, National Institutes of Health, during the conduct of the study; grants from Janssen Pharmaceutica, grants from Merck Sharp & Dohme Corp, grants from ViiV Healthcare, grants from Johnson and Johnson, grants from Pfizer Pharmaceuticals, non-financial support from Kowa Pharmaceuticals America, non-financial support from Sanofi-Aventis, grants from Bristol Myers Squibb, outside the submitted work.
All other authors declare no competing interests.
Figures

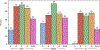


References
-
- World Health Organization (WHO). Consolidated ARV guidelines, June 2013. https://www.who.int/hiv/pub/guidelines/arv2013/art/thirdlineart/en/index.... Accessed: 21-Jan-2019.
-
- World Health Organization (WHO). Antiretroviral medicines in low-and-middle-income countries: forecasts of global and regional demand for 2014–2018: technical report, 2015. https://www.who.int/hiv/pub/amds/arv-forecast2014-2018/en/. Accessed: 21-Jan-2019.
-
- Cambiano V, Bertagnolio S, Jordan MR, et al. Predicted levels of HIV drug resistance: potential impact of expanding diagnosis, retention, and eligibility criteria for antiretroviral therapy initiation. AIDS 2014; 28 Suppl 1: S15–23. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI069423/AI/NIAID NIH HHS/United States
- U01 AI069438/AI/NIAID NIH HHS/United States
- UM1 AI069438/AI/NIAID NIH HHS/United States
- U01 AI038855/AI/NIAID NIH HHS/United States
- UM1 AI069534/AI/NIAID NIH HHS/United States
- R21 MH083308/MH/NIMH NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI069481/AI/NIAID NIH HHS/United States
- U01 AI069432/AI/NIAID NIH HHS/United States
- UM1 AI069399/AI/NIAID NIH HHS/United States
- UM1 AI069453/AI/NIAID NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI069436/AI/NIAID NIH HHS/United States
- UM1 AI069501/AI/NIAID NIH HHS/United States
- U01 AI069423/AI/NIAID NIH HHS/United States
- U01 AI069434/AI/NIAID NIH HHS/United States
- UM1 AI069476/AI/NIAID NIH HHS/United States
- UM1 AI069424/AI/NIAID NIH HHS/United States
- UM1 AI069421/AI/NIAID NIH HHS/United States
- U01 AI069476/AI/NIAID NIH HHS/United States
- U01 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- U01 AI069521/AI/NIAID NIH HHS/United States
- D43 TW010060/TW/FIC NIH HHS/United States
- UM1 AI069521/AI/NIAID NIH HHS/United States
- UM1 AI108568/AI/NIAID NIH HHS/United States
- U01 AI069494/AI/NIAID NIH HHS/United States
- U01 AI069436/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069418/AI/NIAID NIH HHS/United States
- U01 AI046383/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

