Laminin α2 controls mouse and human stem cell behaviour during midbrain dopaminergic neuron development
- PMID: 31371375
- PMCID: PMC6737905
- DOI: 10.1242/dev.172668
Laminin α2 controls mouse and human stem cell behaviour during midbrain dopaminergic neuron development
Abstract
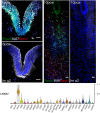
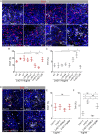
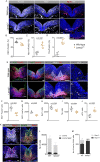
Development of the central nervous system requires coordination of the proliferation and differentiation of neural stem cells. Here, we show that laminin alpha 2 (lm-α2) is a component of the midbrain dopaminergic neuron (mDA) progenitor niche in the ventral midbrain (VM) and identify a concentration-dependent role for laminin α2β1γ1 (lm211) in regulating mDA progenitor proliferation and survival via a distinct set of receptors. At high concentrations, lm211-rich environments maintain mDA progenitors in a proliferative state via integrins α6β1 and α7β1, whereas low concentrations of lm211 support mDA lineage survival via dystroglycan receptors. We confirmed our findings in vivo, demonstrating that the VM was smaller in the absence of lm-α2, with increased apoptosis; furthermore, the progenitor pool was depleted through premature differentiation, resulting in fewer mDA neurons. Examination of mDA neuron subtype composition showed a reduction in later-born mDA neurons of the ventral tegmental area, which control a range of cognitive behaviours. Our results identify a novel role for laminin in neural development and provide a possible mechanism for autism-like behaviours and the brainstem hypoplasia seen in some individuals with mutations of LAMA2.
Keywords: Congenital muscular dystrophy; Dopaminergic neurons; Dystroglycan; Extracellular matrix; Integrin; Laminin; Neural stem cells.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

