Metalloprotease inhibitor TIMP proteins control FGF-2 bioavailability and regulate skeletal growth
- PMID: 31371388
- PMCID: PMC6719459
- DOI: 10.1083/jcb.201906059
Metalloprotease inhibitor TIMP proteins control FGF-2 bioavailability and regulate skeletal growth
Abstract
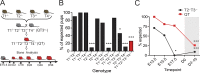
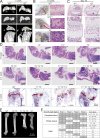
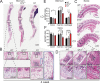
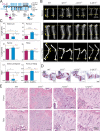
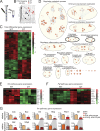
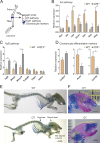
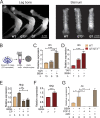
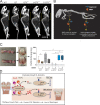
Regulated growth plate activity is essential for postnatal bone development and body stature, yet the systems regulating epiphyseal fusion are poorly understood. Here, we show that the tissue inhibitors of metalloprotease (TIMP) gene family is essential for normal bone growth after birth. Whole-body quadruple-knockout mice lacking all four TIMPs have growth plate closure in long bones, precipitating limb shortening, epiphyseal distortion, and widespread chondrodysplasia. We identify TIMP/FGF-2/IHH as a novel nexus underlying bone lengthening where TIMPs negatively regulate the release of FGF-2 from chondrocytes to allow IHH expression. Using a knock-in approach that combines MMP-resistant or ADAMTS-resistant aggrecans with TIMP deficiency, we uncouple growth plate activity in axial and appendicular bones. Thus, natural metalloprotease inhibitors are crucial regulators of chondrocyte maturation program, growth plate integrity, and skeletal proportionality. Furthermore, individual and combinatorial TIMP-deficient mice demonstrate the redundancy of metalloprotease inhibitor function in embryonic and postnatal development.
© 2019 Saw et al.
Figures


Similar articles
-
Localization of matrix metalloproteinases, (MMPs) their tissue inhibitors, and vascular endothelial growth factor (VEGF) in growth plates of children and adolescents indicates a role for MMPs in human postnatal growth and skeletal maturation.Calcif Tissue Int. 2005 May;76(5):326-35. doi: 10.1007/s00223-004-0161-6. Epub 2005 May 4. Calcif Tissue Int. 2005. PMID: 15868281
-
Epiphyseal abnormalities, trabecular bone loss and articular chondrocyte hypertrophy develop in the long bones of postnatal Ext1-deficient mice.Bone. 2013 Nov;57(1):220-31. doi: 10.1016/j.bone.2013.08.012. Epub 2013 Aug 17. Bone. 2013. PMID: 23958822 Free PMC article.
-
FGF signaling in the osteoprogenitor lineage non-autonomously regulates postnatal chondrocyte proliferation and skeletal growth.Development. 2016 May 15;143(10):1811-22. doi: 10.1242/dev.131722. Epub 2016 Apr 6. Development. 2016. PMID: 27052727 Free PMC article.
-
Matrix metalloproteinases: role in skeletal development and growth plate disorders.Front Biosci. 2006 May 1;11:1702-15. doi: 10.2741/1916. Front Biosci. 2006. PMID: 16368549 Review.
-
Recent Insights into Long Bone Development: Central Role of Hedgehog Signaling Pathway in Regulating Growth Plate.Int J Mol Sci. 2019 Nov 20;20(23):5840. doi: 10.3390/ijms20235840. Int J Mol Sci. 2019. PMID: 31757091 Free PMC article. Review.
Cited by
-
Mechanical forces remodel the cardiac extracellular matrix during zebrafish development.Development. 2024 Jul 1;151(13):dev202310. doi: 10.1242/dev.202310. Epub 2024 Jul 10. Development. 2024. PMID: 38984541 Free PMC article.
-
Genome-Wide Estimates of Runs of Homozygosity, Heterozygosity, and Genetic Load in Two Chinese Indigenous Goat Breeds.Front Genet. 2022 Apr 26;13:774196. doi: 10.3389/fgene.2022.774196. eCollection 2022. Front Genet. 2022. PMID: 35559012 Free PMC article.
-
Unravelling the distinct biological functions and potential therapeutic applications of TIMP2 in cancer.Carcinogenesis. 2022 Jun 4;43(5):405-418. doi: 10.1093/carcin/bgac037. Carcinogenesis. 2022. PMID: 35436325 Free PMC article. Review.
-
Enhanced bone regeneration via endochondral ossification using Exendin-4-modified mesenchymal stem cells.Bioact Mater. 2023 Dec 19;34:98-111. doi: 10.1016/j.bioactmat.2023.12.007. eCollection 2024 Apr. Bioact Mater. 2023. PMID: 38186959 Free PMC article.
-
New developments in the biology of fibroblast growth factors.WIREs Mech Dis. 2022 Jul;14(4):e1549. doi: 10.1002/wsbm.1549. Epub 2022 Feb 9. WIREs Mech Dis. 2022. PMID: 35142107 Free PMC article. Review.
References
-
- Börjesson A.E., Windahl S.H., Karimian E., Eriksson E.E., Lagerquist M.K., Engdahl C., Antal M.C., Krust A., Chambon P., Sävendahl L., and Ohlsson C.. 2012. The role of estrogen receptor-α and its activation function-1 for growth plate closure in female mice. Am. J. Physiol. Endocrinol. Metab. 302:E1381–E1389. 10.1152/ajpendo.00646.2011 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

