A Receptor Tyrosine Kinase Plays Separate Roles in Sensory Integration and Associative Learning in C. elegans
- PMID: 31371455
- PMCID: PMC6712205
- DOI: 10.1523/ENEURO.0244-18.2019
A Receptor Tyrosine Kinase Plays Separate Roles in Sensory Integration and Associative Learning in C. elegans
Abstract
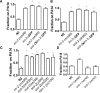
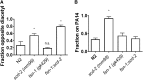
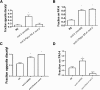
Associative learning and sensory integration are two behavioral processes that involve the sensation and processing of stimuli followed by an altered behavioral response to these stimuli, with learning requiring memory formation and retrieval. We found that the cellular and molecular actions of scd-2 dissociate sensory integration and associative learning. This was discovered through investigation of a Caenorhabditis elegans mutation (lrn-2 (mm99)) affecting both processes. After mapping and sequencing, lrn-2 was found to be allelic to the gene, scd-2scd-2-mediated associative learning and sensory integration operate in separate neurons as separate processes. We also find that memories can form from associations that are processed and stored independently from the integration of stimuli preceding an immediate behavioral decision.
Keywords: C. elegans; PA14; associative learning; chemotaxis; sensory integration.
Copyright © 2019 Wolfe et al.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
