Building a synthetic mechanosensitive signaling pathway in compartmentalized artificial cells
- PMID: 31371493
- PMCID: PMC6708380
- DOI: 10.1073/pnas.1903500116
Building a synthetic mechanosensitive signaling pathway in compartmentalized artificial cells
Abstract
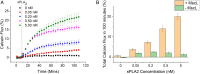
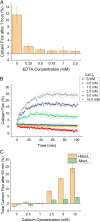
To date, reconstitution of one of the fundamental methods of cell communication, the signaling pathway, has been unaddressed in the bottom-up construction of artificial cells (ACs). Such developments are needed to increase the functionality and biomimicry of ACs, accelerating their translation and application in biotechnology. Here, we report the construction of a de novo synthetic signaling pathway in microscale nested vesicles. Vesicle-cell models respond to external calcium signals through activation of an intracellular interaction between phospholipase A2 and a mechanosensitive channel present in the internal membranes, triggering content mixing between compartments and controlling cell fluorescence. Emulsion-based approaches to AC construction are therefore shown to be ideal for the quick design and testing of new signaling networks and can readily include synthetic molecules difficult to introduce to biological cells. This work represents a foundation for the engineering of multicompartment-spanning designer pathways that can be utilized to control downstream events inside an AC, leading to the assembly of micromachines capable of sensing and responding to changes in their local environment.
Keywords: MscL; artificial cells; nested vesicle; phospholipase A2; signaling pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lai E. C., Notch signaling: Control of cell communication and cell fate. Development 131, 965–973 (2004). - PubMed
-
- Waters C. M., Bassler B. L., Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21, 319–346 (2005). - PubMed
-
- Ratajczak J., Wysoczynski M., Hayek F., Janowska-Wieczorek A., Ratajczak M. Z., Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 20, 1487–1495 (2006). - PubMed
-
- Rasmussen H., Cell communication, calcium ion, and cyclic adenosine monophosphate. Science 170, 404–412 (1970). - PubMed
-
- Clapham D. E., Calcium signaling. Cell 131, 1047–1058 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

