Identification of key enzymes responsible for protolimonoid biosynthesis in plants: Opening the door to azadirachtin production
- PMID: 31371503
- PMCID: PMC6708365
- DOI: 10.1073/pnas.1906083116
Identification of key enzymes responsible for protolimonoid biosynthesis in plants: Opening the door to azadirachtin production
Abstract
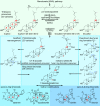
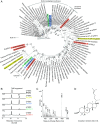
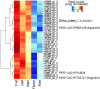
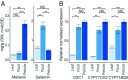
Limonoids are natural products made by plants belonging to the Meliaceae (Mahogany) and Rutaceae (Citrus) families. They are well known for their insecticidal activity, contribution to bitterness in citrus fruits, and potential pharmaceutical properties. The best known limonoid insecticide is azadirachtin, produced by the neem tree (Azadirachta indica). Despite intensive investigation of limonoids over the last half century, the route of limonoid biosynthesis remains unknown. Limonoids are classified as tetranortriterpenes because the prototypical 26-carbon limonoid scaffold is postulated to be formed from a 30-carbon triterpene scaffold by loss of 4 carbons with associated furan ring formation, by an as yet unknown mechanism. Here we have mined genome and transcriptome sequence resources for 3 diverse limonoid-producing species (A. indica, Melia azedarach, and Citrus sinensis) to elucidate the early steps in limonoid biosynthesis. We identify an oxidosqualene cyclase able to produce the potential 30-carbon triterpene scaffold precursor tirucalla-7,24-dien-3β-ol from each of the 3 species. We further identify coexpressed cytochrome P450 enzymes from M. azedarach (MaCYP71CD2 and MaCYP71BQ5) and C. sinensis (CsCYP71CD1 and CsCYP71BQ4) that are capable of 3 oxidations of tirucalla-7,24-dien-3β-ol, resulting in spontaneous hemiacetal ring formation and the production of the protolimonoid melianol. Our work reports the characterization of protolimonoid biosynthetic enzymes from different plant species and supports the notion of pathway conservation between both plant families. It further paves the way for engineering crop plants with enhanced insect resistance and producing high-value limonoids for pharmaceutical and other applications by expression in heterologous hosts.
Keywords: insecticides; limonoids; natural products; neem; terpenes.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: Authors involved in patent filing are H.H., M.J.S., and A.O.
Figures

References
-
- Morgan E. D., Azadirachtin, a scientific gold mine. Bioorg. Med. Chem. 17, 4096–4105 (2009). - PubMed
-
- Tan Q.-G., Luo X.-D., Meliaceous limonoids: Chemistry and biological activities. Chem. Rev. 111, 7437–7522 (2011). - PubMed
-
- Roy A., Saraf S., Limonoids: Overview of significant bioactive triterpenes distributed in plants kingdom. Biol. Pharm. Bull. 29, 191–201 (2006). - PubMed
-
- Zhang Y. Y., Xu H., Recent progress in the chemistry and biology of limonoids. RSC Adv. 7, 35191–35220 (2017).
-
- Arnott S., Davie A. W., Robertson J. M., Sim G. A., Watson D. G., The structure of limonin. Experientia 16, 49–51 (1960).
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- BBS/E/J/000PR9790/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R01 GM121527/GM/NIGMS NIH HHS/United States
- BBS/E/J/00000614/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/L014130/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- DP2 AT008321/AT/NCCIH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

