Phosphatidylserine flipping by the P4-ATPase ATP8A2 is electrogenic
- PMID: 31371510
- PMCID: PMC6697784
- DOI: 10.1073/pnas.1910211116
Phosphatidylserine flipping by the P4-ATPase ATP8A2 is electrogenic
Abstract
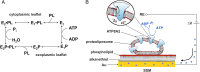
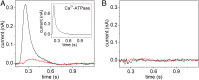
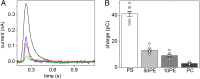
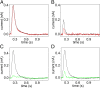
Phospholipid flippases (P4-ATPases) utilize ATP to translocate specific phospholipids from the exoplasmic leaflet to the cytoplasmic leaflet of biological membranes, thus generating and maintaining transmembrane lipid asymmetry essential for a variety of cellular processes. P4-ATPases belong to the P-type ATPase protein family, which also encompasses the ion transporting P2-ATPases: Ca2+-ATPase, Na+,K+-ATPase, and H+,K+-ATPase. In comparison with the P2-ATPases, understanding of P4-ATPases is still very limited. The electrogenicity of P4-ATPases has not been explored, and it is not known whether lipid transfer between membrane bilayer leaflets can lead to displacement of charge across the membrane. A related question is whether P4-ATPases countertransport ions or other substrates in the opposite direction, similar to the P2-ATPases. Using an electrophysiological method based on solid supported membranes, we observed the generation of a transient electrical current by the mammalian P4-ATPase ATP8A2 in the presence of ATP and the negatively charged lipid substrate phosphatidylserine, whereas only a diminutive current was generated with the lipid substrate phosphatidylethanolamine, which carries no or little charge under the conditions of the measurement. The current transient seen with phosphatidylserine was abolished by the mutation E198Q, which blocks dephosphorylation. Likewise, mutation I364M, which causes the neurological disorder cerebellar ataxia, mental retardation, and disequilibrium (CAMRQ) syndrome, strongly interfered with the electrogenic lipid translocation. It is concluded that the electrogenicity is associated with a step in the ATPase reaction cycle directly involved in translocation of the lipid. These measurements also showed that no charged substrate is being countertransported, thereby distinguishing the P4-ATPase from P2-ATPases.
Keywords: CAMRQ syndrome; SSM method; charge displacement; flippase; phospholipid transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bevers E. M., Williamson P. L., Getting to the outer leaflet: Physiology of phosphatidylserine exposure at the plasma membrane. Physiol. Rev. 96, 605–645 (2016). - PubMed
-
- Puts C. F., Holthuis J. C., Mechanism and significance of P4 ATPase-catalyzed lipid transport: Lessons from a Na+/K+-pump. Biochim. Biophys. Acta 1791, 603–611 (2009). - PubMed
-
- Kaplan J. H., Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 71, 511–535 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

