Rhodopsin-based voltage imaging tools for use in muscles and neurons of Caenorhabditis elegans
- PMID: 31371514
- PMCID: PMC6708366
- DOI: 10.1073/pnas.1902443116
Rhodopsin-based voltage imaging tools for use in muscles and neurons of Caenorhabditis elegans
Abstract
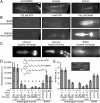
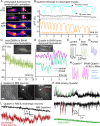
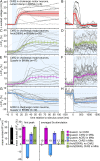
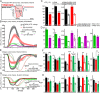
Genetically encoded voltage indicators (GEVIs) based on microbial rhodopsins utilize the voltage-sensitive fluorescence of all-trans retinal (ATR), while in electrochromic FRET (eFRET) sensors, donor fluorescence drops when the rhodopsin acts as depolarization-sensitive acceptor. In recent years, such tools have become widely used in mammalian cells but are less commonly used in invertebrate systems, mostly due to low fluorescence yields. We systematically assessed Arch(D95N), Archon, QuasAr, and the eFRET sensors MacQ-mCitrine and QuasAr-mOrange, in the nematode Caenorhabditis elegans ATR-bearing rhodopsins reported on voltage changes in body wall muscles (BWMs), in the pharynx, the feeding organ [where Arch(D95N) showed approximately 128% ΔF/F increase per 100 mV], and in neurons, integrating circuit activity. ATR fluorescence is very dim, yet, using the retinal analog dimethylaminoretinal, it was boosted 250-fold. eFRET sensors provided sensitivities of 45 to 78% ΔF/F per 100 mV, induced by BWM action potentials, and in pharyngeal muscle, measured in simultaneous optical and sharp electrode recordings, MacQ-mCitrine showed approximately 20% ΔF/F per 100 mV. All sensors reported differences in muscle depolarization induced by a voltage-gated Ca2+-channel mutant. Optogenetically evoked de- or hyperpolarization of motor neurons increased or eliminated action potential activity and caused a rise or drop in BWM sensor fluorescence. Finally, we analyzed voltage dynamics across the entire pharynx, showing uniform depolarization but compartmentalized repolarization of anterior and posterior parts. Our work establishes all-optical, noninvasive electrophysiology in live, intact C. elegans.
Keywords: all-optical electrophysiology; electrochromic FRET; microbial rhodopsin; neuromuscular; voltage imaging.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Knöpfel T., Genetically encoded optical indicators for the analysis of neuronal circuits. Nat. Rev. Neurosci. 13, 687–700 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

